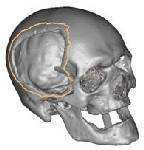
CT scanned data converted to CADCAM
data showing damaged area due to trama.
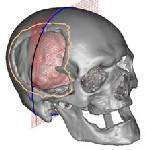
Utilization of CADCAM data to drive
cutter path for CNC machines.
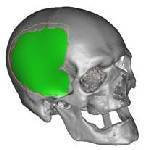
Fit and Form check of finished piece
that replaces the damaged bone structure.
LITTLE ROCK, AR--(Marketwire - April 28, 2009) - Kelyniam Global, Inc. (PINKSHEETS: KLYG), an advanced Engineering and Rapid Prototyping Company specializing in the use of CADCAM technology, announced today that the company will become a Source Supplier of Custom Cranial Implants and Maxillofacial structures using our engineering expertise and knowledge of high tolerance manufacturing and advance polymers.
Kelyniam announced in a press release dated November 10, 2008, that the cranial implant process the company was involved with was under FDA inspection. Since this announcement, the company has decided to create an entire process of its own, removing all supply line vendors and increasing revenue generation and profitability.
James Ketner, President and CEO of Kelyniam Global, Inc., stated: "I would like to take some time to update the shareholders on why this decision was made, and what happened to cause us to go in this direction. Kelyniam's previous role in the Cranial Implant process was to provide a Tier 1 supplier with models generated from CT data that were a near perfect replication of the area in the skull to be replaced. Those models were used by this Tier 1 supplier to create a mold in which a Bio-Compatible Material was injected that was then going to be the actual piece that went into the human body. This Bio-Compatible Material then needed to be sterilized before shipping to a major medical supplier, and that medical supplier then shipped the finished product to the hospital, which went into the OR and was implanted into the patient. The medical products supplier that we were supplying encountered some difficulties in the sterilization process which required further inspection by the FDA. These problems had nothing at all to do with our product. In general, we fixed the problems by taking on the entire process cradle to grave, and by eliminating any other manufactures in the process. We will have the ability to sell directly to the Doctors, or have the possibility of contracting a major medical supplier.
This new process will be the following:
1. Receipt of CT Scanned Data from Hospital.
2. Translate CT Scanned Data into CADCAM Data.
3. We are going to machine the actual piece that goes into the body by the use of computerized numeric control (CNC), eliminating the necessity for creating a mold. The material we are going to use in the machining process is an FDA approved, PEEK (polyaryletheretherketone), extremely high grade polymer with temperature properties which will allow us to sterilize by the use of steam and pressure instead of Ethylene Oxide which the FDA was having a problem with.
4. By the use of Stereolithography, we will create a "Host Bone," i.e. the undamaged part of the body, to use as a "Go, No Go" check gage which will be inspected using our advanced laser scanner against the CT Scanned Data supplied to us by the hospital, and the replacement bone piece will be checked against the mating host bone.
5. The sterilized machined implant will go directly to the OR, accompanied by the inspected host bone and laser scanned inspection data, and will then be installed into the patient.
We are going to initially out-source the sterilization process in order to reduce the lead time for this new process to become operational. We are expecting this process to be up and running in 45-60 days after this announcement. By removing any suppliers in the implant process and by Kelyniam undertaking the entire process cradle to grave, the time it takes from scan to the delivery of the implant will be reduced. We also then have nobody to blame for the success or failure of our products due to quality issues except ourselves."
Kelyniam created approximately 350 cranial implant models in 9 months of 2008 and is anticipating a conservative estimate to be able to produce 460 of these per year based on 2008 historic production of 38 units per month. The company is also investigating the use of the FDA approved polymers and this type of production method in all aspects of artificial bone replacements throughout the human body.
A study, using a well developed in vitro model published by researchers at the University of Connecticut, observed differentiated bone cells and mineralization upon the PEEK-OPTIMA surfaces. This latest observation, along with the growing number of published findings using the polymer, suggests that PEEK-OPTIMA may lend itself to Osseointegration -- the direct connection between the bone and implant surface. www.azom.com/news.asp?newsID=8284
Kelyniam Global, Inc. recently posted a video which explains more about the company's new and exciting medical process utilizing CADCAM technology. Please take in consideration that the process discussed in the video has now changed somewhat, and Kelyniam now produces the end product. The video can be watched by going to the company website, and selecting the "Watch our New Medical Video" on Kelyniam's home page, or by following the link www.kelyniam.com/video.wmv
About Kelyniam Global, Incorporated:
Kelyniam Global, Inc. service a vast array of clients across numerous fields and industries encompassing, but not limited to, medical, automotive, aerospace, jewelry, nautical and consumer products. The company specializes in the use of CADCAM technology with recent developments in the production of high precision replication of artificial bone implants for medical applications.
More information about Kelyniam Global, Inc. can be found by following the
links provided on our home page. www.kelyniam.com
Contact:
John Mastoloni VP/Sales
Engineering Division
Kelyniam Global, Inc.
www.kelyniam.com
1-860-832-9331 X221




