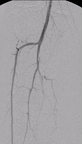
Summa Therapeutics, LLC announced that the first-in-man injectable angioplasty procedures for patients with below-knee peripheral arterial disease were performed successfully using its Finesse InjectableTM balloon catheter platform.
First In-Man Experience Exceeds Expectations, Cited as “Game Changer” for Below-the-Knee Procedures
CAMBRIDGE, Mass.--(BUSINESS WIRE)-- Summa Therapeutics, LLC announced today that the first-in-man injectable angioplasty procedures for patients with below-knee peripheral arterial disease (PAD) were performed successfully using its Finesse InjectableTM balloon catheter platform.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20240117168525/en/
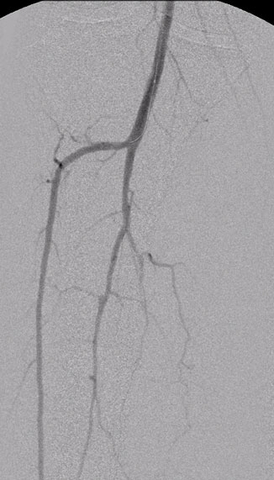
Figure 1. Calf angiogram obtained using the Summa Finesse catheter with 1 ml equivalent of 60% contrast. (Photo: Business Wire)
The Summa Finesse Injectable catheter is the industry’s first hybrid diagnostic and therapeutic angioplasty balloon catheter developed to facilitate treatment of patients at risk of limb loss due to below-knee PAD. It was designed to improve the efficiency of peripheral angioplasty procedures by reducing equipment and contrast needs.
Each year, there are more than 140,000 lower extremity amputations in the U.S., most due to circulation issues. For patients at risk of amputation due to poor circulation, angioplasty procedures below the knee can be limb-saving. However, angioplasty procedures below the knee are complex and typically require numerous exchanges of specialized equipment during a procedure.
The Summa Finesse Injectable platform is designed to reduce the need for some cumbersome catheter exchanges through its ability to provide contrast injection on the fly, enabling users to move seamlessly from injection to balloon inflation throughout complex procedures. This distinct operator convenience streamlines the procedure and reduces the need for equipment. All users reported that the injection ability exceeded expectations.
Angioplasty procedures below the knee also require x-ray contrast, which can cause damage to kidney function. This is an especially critical issue because many patients undergoing angioplasty procedures below the knee have diabetes and kidney dysfunction. Operators reported that the Summa Finesse Injectable catheter allowed for decreased use of x-ray contrast, with 100% of procedures requiring less contrast than typically used with conventional devices. In the calf, ultra-low contrast angiograms were obtained with as little as 1 ml equivalent of contrast (see figure 1 below).
The first-in-man procedures were done at Mt. Sinai hospital in New York City by Drs. Rahul Patel and Robert Lookstein, and at the Advanced Vascular and Interventional Services office-base laboratory in West Orange, New Jersey by Drs. John Rundback and Kevin Herman.
“The Summa balloon platform is a game changer that provides several unique advantages,” said Dr. Rundback, award-winning interventional radiologist and vascular specialist. “In our busy limb salvage practice, it will be an important tool.”
The Summa Finesse Injectable is a multifunctional catheter that serves as a crossing catheter, diagnostic angiography catheter, and angioplasty catheter to reduce equipment and contrast needs. It was noted that in many cases with the Summa Finesse catheter functioning as a crossing catheter, microcatheters were not needed to cross arterial obstructions.
“Summa’s system makes below-knee angioplasty cases easier compared to a conventional setup,” said Dr. Herman, nationally acclaimed interventional radiologist. “I start with Finesse and use it to perform angiography, cross lesions, and do serial angioplasties of multiple arterial segments in the calf -- all with the same catheter. It’s a better designed system for below-knee angioplasty.”
“In the first cases, expectations were met or exceeded, including improved procedural efficiency and lower equipment needs,” said Dr. Timothy Murphy, CEO, Summa Therapeutics. “All users reported using less contrast and yet angiographic quality was described as excellent. The Summa Finesse Injectable has tremendous market potential and may become the standard of care when treating patients with chronic kidney disease, for whom contrast sparing is paramount.”
The first-in-man experience represents a significant clinical milestone for Summa Therapeutics, a leading innovator in the cardiovascular medical device industry. The Summa Finesse Injectable is the first hybrid catheter launched in the planned line of interventional radiology devices from Summa.
About Summa Therapeutics
Summa Therapeutics, LLC is a commercial-stage medical device company specializing in the development of innovative solutions for the field of cardiovascular interventional medicine. With a strong focus on improving patient outcomes, Summa Therapeutics, LLC is dedicated to delivering medical technologies that address critical unmet needs in peripheral vascular disease care. For more information about the Summa Finesse angioplasty balloon product line or to inquire about distribution, please visit summatherapeutics.com or contact Summa at (877) 265-2685.
View source version on businesswire.com: https://www.businesswire.com/news/home/20240117168525/en/
Contacts
Media:
Erica Alejaldre
Point B Partners
954-268-9692
erica@pointbpartners.com
Source: Summa Therapeutics, LLC
Figure 1. Calf angiogram obtained using the Summa Finesse catheter with 1 ml equivalent of 60% contrast. (Photo: Business Wire)
Dr. Rundback (second from left) and team from AIVS in West Orange, NJ. (Photo: Business Wire)
View this news release and multimedia online at:
http://www.businesswire.com/news/home/20240117168525/en