Highly Statistically Significant Results (P<0.001) Demonstrated on Primary Composite Endpoint and All Secondary Endpoints
DUBLIN, Nov. 17, 2016 /PRNewswire/ -- Endo International plc (NASDAQ / TSX: ENDP) announced today positive results from its Phase 2b study of collagenase clostridium histolyticum (or "CCH") for the treatment of edematous fibrosclerotic panniculopathy ("EFP"), commonly known as cellulite. CCH is known in its currently approved indications in the U.S. as XIAFLEX® for adult Dupuytren's contracture and Peyronie's disease. Trial subjects receiving CCH showed statistically significant levels of improvement in the appearance of cellulite with treatment, as measured by the trial's primary endpoint (p<0.001), compared to those subjects receiving placebo. CCH was well-tolerated in the actively treated subjects with most adverse events (AEs) being mild to moderate in severity, and primarily limited to the local injection area.
"We continue to be encouraged and very excited by the promising results of our CCH program, especially in the cellulite indication a condition which affects many millions of people," said Dr. Susan Hall, Executive Vice President, Chief Scientific Officer and Global Head of R&D at Endo. "We believe these data further reinforce our belief that CCH could be a potential treatment option for those with cellulite and we look forward to working with FDA to efficiently and effectively advance our development of the program into Phase 3."
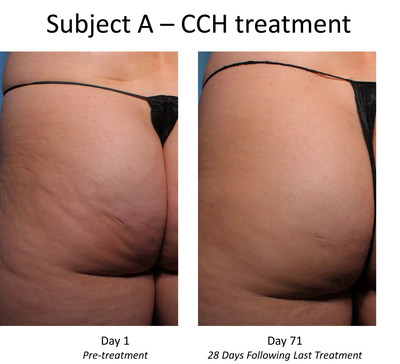
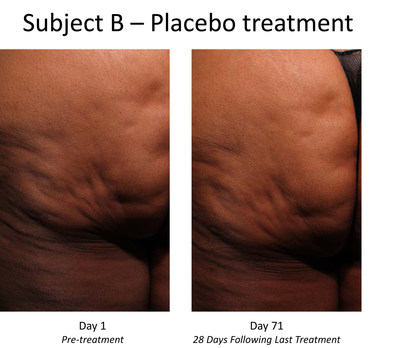
The Phase 2b trial enrolled 375 women with moderate or severe cellulite aged 18 years or older in the United States. Each subject received up to three treatment sessions of CCH (0.84 mg / session) or placebo with each treatment session occurring approximately 21 days apart. Twelve injections were administered into cellulite dimples during each session across an entire treatment quadrant left or right buttock or left or right posteriolateral thigh. At both the outset and conclusion of the study period (28 days after the last treatment), cellulite severity was assessed by each patient and clinician using two photonumeric cellulite severity scales developed by Endo and third-party experts. The scales the Photonumeric Cellulite Severity Scale (PCSS) are 5-point scales ranging from 0 (no cellulite) to 4 (severe cellulite) that measure improvement in the appearance of cellulite.
The Phase 2b trial's primary endpoint was the proportion of composite responders at Day 71 defined as subjects with a 2-point improvement in severity from baseline in the clinician-reported (CR) PCSS and a 2-point improvement in the patient-reported (PR) PCSS. Additional endpoints include a composite of 1-point responders, the percentage of responders with 1-point and 2-point improvements on the CR-PCSS and PR-PCSS, assessment of improvement by patient and clinician using the Global Aesthetic Improvement Scale (GAIS); subject satisfaction, and change in the Hexsel cellulite severity scale.
"I believe these positive results demonstrate that CCH has the potential to be a treatment option for cellulite, a condition for which there are very limited safe and proven effective treatments," said Mitchel P. Goldman, MD, Medical Director, Cosmetic Laser Dermatology. "With so many millions of women affected by cellulite, there is a substantial need for new and innovative therapeutic options."
Key Phase 2b Trial Results Include:
- Subjects receiving CCH demonstrated a highly statistically significant improvement in the primary endpoint of composite investigators' and patients' assessments of the appearance of cellulite, as measured by a two-point improvement in both the CR-PCSS and PR-PCSS scores, with a p-value of <0.001 versus placebo
- Subjects receiving CCH demonstrated a highly statistically significant improvement in the composite investigators' and patients' assessments of the appearance of cellulite, as measured by a one-point improvement in both the CR-PCSS and PR-PCSS scores, with a p-value of <0.001 versus placebo
- A highly significant proportion of CCH subjects reported being "Satisfied" or "Very Satisfied" with their cellulite treatment, compared to placebo subjects, with a p-value of <0.001
- A highly significant proportion of CCH subjects were reported as "Improved" or "Very Improved" or "Very Much Improved" in global appearance of their cellulite area as assessed by the subjects and investigators, compared to placebo subjects, with a p-value of <0.001
- CCH was well-tolerated by all dose groups with most adverse events (AEs) being mild to moderate and primarily limited to the local injection area; 92 percent of all related AEs were mild to moderate in the CCH group compared to 96 percent in the placebo group; the most common AEs were expected and included injection site bruising (approximately 75 percent) and injection site pain (approximately 59 percent)
"These data are exciting for the medical community and definitely warrant further investigation and development," said Neil Sadick, MD, Clinical Professor of Dermatology, Weill Cornell Medical College. "By successfully advancing this program, we could offer patients currently seeking cellulite treatment a new and promising option."
Results for an earlier Phase 2a trial of CCH for the treatment of cellulite demonstrated that three doses of CCH (low (0.06mg), mid (0.48mg) and high (0.84mg)) showed an improvement in the appearance of cellulite as measured by the trial endpoints of an investigator and a patient score on the GAIS, which was adapted for use in cellulite. The mid and high dose groups demonstrated a statistically significant improvement in the appearance of cellulite, as measured by GAIS scores, with a p-value of <0.05 compared to placebo for both endpoints. In the mid and high dose groups, 68 percent of patients reported being "Satisfied" or "Very Satisfied" with the results of their treatment, compared to only 34 percent of patients randomized to placebo. CCH was well-tolerated by all dose groups with most adverse events (AEs) being mild to moderate and primarily limited to the local injection area.
About Cellulite
Cellulite is a localized metabolic disorder of tissue under the skin that has been reported in 85 to 98 percent of post-pubertal females and affects women of all races and ethnicities[i] [ii]. The condition can involve the loss of elasticity or shrinking of collagen cords, called "septae," that attach the skin to the muscle layers below. When fat in cellulite prone areas swells and expands, the septae tether the skin, which causes the surface dimpling characteristic of cellulite[iii]. CCH is intended to target and lyse, or break, those collagen tethers with the goal of releasing the skin dimpling and potentially resulting in smoothing of the skin. Despite multiple therapeutic approaches for the attempted treatment of cellulite, there are no FDA-approved pharmacological treatments and little scientific evidence that any current treatments are beneficial[iv].
About XIAFLEX
XIAFLEX® (collagenase clostridium histolyticum, or CCH) is a biologic approved in the U.S., EU, Canada, Australia and Japan for the treatment of adult Dupuytren's contracture (DC) patients with a palpable cord and in the U.S. and EU for the treatment of adult men with Peyronie's disease (PD) with a palpable plaque and penile curvature deformity of at least 30 degrees at the start of therapy. XIAFLEX® consists of a combination of two subtypes of collagenase, derived from Clostridium histolyticum. Together, the collagenase subtypes are thought to work synergistically to break the bonds of the collagen structure. XIAFLEX® has been granted Orphan status in the U.S. by the FDA for DC and PD.
IMPORTANT SAFETY INFORMATION FOR XIAFLEX® (DUPUYTREN'S CONTRACTURE)
INDICATION
XIAFLEX® is indicated for the treatment of adult patients with Dupuytren's contracture with a palpable cord.
IMPORTANT SAFETY INFORMATION FOR XIAFLEX®
- XIAFLEX® is contraindicated in patients with a history of hypersensitivity to XIAFLEX® or to collagenase used in any other therapeutic application or application method
- In the controlled and uncontrolled portions of clinical trials in Dupuytren's contracture, flexor tendon ruptures occurred after XIAFLEX® injection. Injection of XIAFLEX® into collagen-containing structures such as tendons or ligaments of the hand may result in damage to those structures and possible permanent injury such as tendon rupture or ligament damage. Therefore, XIAFLEX® should be injected only into the collagen cord with a MP or PIP joint contracture, and care should be taken to avoid injecting into tendons, nerves, blood vessels, or other collagen-containing structures of the hand. When injecting a cord affecting a PIP joint of the fifth finger, the needle insertion should not be more than 2 to 3 mm in depth and avoid injecting more than 4 mm distal to the palmar digital crease
- Other XIAFLEX®-associated serious local adverse reactions in the controlled and uncontrolled portions of the studies included pulley rupture, ligament injury, complex regional pain syndrome (CRPS), sensory abnormality of the hand, and skin laceration (tear). In a historically controlled post-marketing trial, the incidence of skin laceration (22%) was higher for subjects treated with two concurrent injections of XIAFLEX® compared with subjects treated with up to three single injections in the placebo-controlled premarketing trials (9%). Cases of skin laceration requiring skin graft after finger extension procedures have been reported post-marketing. Signs or symptoms that may reflect serious injury to the injected finger/hand should be promptly evaluated because surgical intervention may be required
- In the controlled portions of the clinical trials in Dupuytren's contracture, a greater proportion of XIAFLEX®-treated patients (15%) compared to placebo-treated patients (1%) had mild allergic reactions (pruritus) after up to 3 injections. The incidence of XIAFLEX®-associated pruritus increased after more XIAFLEX® injections in patients with Dupuytren's contracture
- Because XIAFLEX® contains foreign proteins, severe allergic reactions to XIAFLEX® can occur. Anaphylaxis was reported in a post-marketing clinical study in one patient who had previous exposure to XIAFLEX® for the treatment of Dupuytren's contracture. Healthcare providers should be prepared to address severe allergic reactions following XIAFLEX® injections
- In the XIAFLEX® trials in Dupuytren's contracture, 70% and 38% of XIAFLEX®-treated patients developed an ecchymosis/contusion or an injection site hemorrhage, respectively. Patients with abnormal coagulation (except for patients taking low-dose aspirin, eg, up to 150 mg per day) were excluded from participating in these studies. Therefore, the efficacy and safety of XIAFLEX® in patients receiving anticoagulant medications (other than low-dose aspirin, eg, up to 150 mg per day) within 7 days prior to XIAFLEX® administration is not known. In addition, it is recommended to avoid use of XIAFLEX® in patients with coagulation disorders, including patients receiving concomitant anticoagulants (except for low-dose aspirin)
- In the XIAFLEX® clinical trials for Dupuytren's contracture, the most common adverse reactions reported in 25% of patients treated with XIAFLEX® and at an incidence greater than placebo were edema peripheral (eg, swelling of the injected hand), contusion, injection site hemorrhage, injection site reaction, and pain in the injected extremity
IMPORTANT SAFETY INFORMATION FOR XIAFLEX® (PEYRONIE'S DISEASE)
INDICATION
XIAFLEX® is indicated for the treatment of adult men with Peyronie's disease with a palpable plaque and curvature deformity of at least 30 degrees at the start of therapy.
IMPORTANT SAFETY INFORMATION FOR XIAFLEX®
WARNING: CORPORAL RUPTURE (PENILE FRACTURE) OR OTHER SERIOUS PENILE INJURY IN THE TREATMENT OF PEYRONIE'S DISEASE
Corporal rupture (penile fracture) was reported as an adverse reaction in 5 of 1044 (0.5%) XIAFLEX®-treated patients in clinical studies. In other XIAFLEX®-treated patients (9 of 1044; 0.9%), a combination of penile ecchymoses or hematoma, sudden penile detumescence, and/or a penile "popping" sound or sensation was reported, and in these cases, a diagnosis of corporal rupture cannot be excluded. Severe penile hematoma was also reported as an adverse reaction in 39 of 1044 (3.7%) XIAFLEX®-treated patients.
Signs or symptoms that may reflect serious penile injury should be promptly evaluated to assess for corporal rupture or severe penile hematoma which may require surgical intervention.
Because of the risks of corporal rupture or other serious penile injury, XIAFLEX® is available for the treatment of Peyronie's disease only through a restricted program under a Risk Evaluation and Mitigation Strategy (REMS) called the XIAFLEX® REMS Program.
- XIAFLEX® is contraindicated in the treatment of Peyronie's plaques that involve the penile urethra due to potential risk to this structure and in patients with a history of hypersensitivity to XIAFLEX® or to collagenase used in any other therapeutic application or application method
- Injection of XIAFLEX® into collagen-containing structures such as the corpora cavernosa of the penis may result in damage to those structures and possible injury such as corporal rupture (penile fracture). Therefore, XIAFLEX® should be injected only into the Peyronie's plaque and care should be taken to avoid injecting into the urethra, nerves, blood vessels, corpora cavernosa or other collagen-containing structures of the penis
- In the double-blind, placebo-controlled portions of the clinical trials in Peyronie's disease, a greater proportion of XIAFLEX®-treated patients (4%) compared to placebo-treated patients (1%) had localized pruritus after up to 4 treatment cycles (involving up to 8 XIAFLEX® injection procedures). The incidence of XIAFLEX®-associated pruritus was similar after each injection regardless of the number of injections administered
- Because XIAFLEX® contains foreign proteins, severe allergic reactions to XIAFLEX® can occur. Anaphylaxis was reported in a post-marketing clinical trial in one patient who had previous exposure to XIAFLEX® for the treatment of Dupuytren's contracture. Healthcare providers should be prepared to address severe allergic reactions following XIAFLEX® injections. The safety of more than one treatment course of XIAFLEX® is not known
- In the XIAFLEX® controlled trials in Peyronie's disease, 65.5% of XIAFLEX®-treated patients developed penile hematoma, and 14.5% developed penile ecchymosis. Patients with abnormal coagulation (except for patients taking low-dose aspirin, eg, up to 150 mg per day) were excluded from participating in these studies. Therefore, the efficacy and safety of XIAFLEX® in patients receiving anticoagulant medications (other than low-dose aspirin, eg, up to 150 mg per day) within 7 days prior to XIAFLEX® administration is not known. In addition, it is recommended to avoid use of XIAFLEX® in patients with coagulation disorders, including patients receiving concomitant anticoagulants (except for low-dose aspirin)
- In the XIAFLEX® clinical trials for Peyronie's disease, the most frequently reported adverse drug reactions (25%) and at an incidence greater than placebo included: penile hematoma, penile swelling, and penile pain
Please see the full Prescribing Information, including Boxed Warning and Medication Guide, available at www.XIAFLEX.com.
About Endo International plc
Endo International plc (NASDAQ / TSX: ENDP) is a global specialty pharmaceutical company focused on improving patients' lives while creating shareholder value. Endo develops, manufactures, markets and distributes quality branded and generic pharmaceutical products as well as over-the-counter medications though its operating companies. Endo has global headquarters in Dublin, Ireland, and U.S. headquarters in Malvern, PA. Learn more at www.endo.com.
Cautionary Note Regarding Forward-Looking Statements
This press release contains "forward-looking statements," including, but not limited to, the statements by Drs. Hall, Sadick and Goldman. These statements are based on current expectations of future events. If underlying assumptions prove inaccurate or unknown risks or uncertainties materialize, actual results could vary materially from Endo's expectations and projections. Risks and uncertainties include, among other things, general industry and market conditions; technological advances and patents attained by competitors; challenges inherent in the research and development and regulatory processes; challenges related to product marketing, such as the unpredictability of market acceptance for new products and/or the acceptance of new indications for such products; inconsistency of treatment results among patients; potential difficulties in manufacturing; general economic conditions; and governmental laws and regulations affecting domestic and foreign operations. Endo expressly disclaims any intent or obligation to update these forward-looking statements except as required by law. Additional information concerning these and other risk factors can be found in Endo's periodic reports filed with the U.S. Securities and Exchange Commission and in Canada on the System for Electronic Data Analysis and Retrieval ("SEDAR"), including current reports on Form 8-K, quarterly reports on Form 10-Q and annual reports on Form 10-K. Additional information about Endo is available on the World Wide Web at www.endo.com or you can contact the Endo Investor Relations department by calling (484) 216-0000.
[i] Avram, Cellulite: a review of its physiology and treatment, Journal of Cosmetic Laser Therapy 2004; 6: 181185.
[ii] Khan MH et al. Treatment of cellulite: Part I. Pathophysiology. J Am Acad Dermatol. 2010 Mar;62(3):361-70.
[iii] Querleux, Anatomy and physiology of subcutaneous adipose tissue by in vivo MRI and spectroscopy: Relationship with sex and presence of cellulite, Skin Research and Technology; 8: 118-124.
[iv] Wanner M et al. An evidence-based assessment of treatments for cellulite. J Drugs Dermatol. 2008 Apr;7(4):341-5.
Photo - http://photos.prnewswire.com/prnh/20161116/440530
Photo - http://photos.prnewswire.com/prnh/20161116/440497
To view the original version on PR Newswire, visit:http://www.prnewswire.com/news-releases/endo-announces-positive-data-from-phase-2b-study-of-collagenase-clostridium-histolyticum-cch-in-patients-with-cellulite-300364763.html
SOURCE Endo International plc





