Edwards Receives FDA Clearance For Advanced Noninvasive Monitoring System
ClearSight system enables clinicians to reduce risk of post-surgical complications
IRVINE, Calif., June 30, 2014 /PRNewswire/ -- Edwards Lifesciences Corporation (NYSE: EW), the global leader in the science of heart valves and hemodynamic monitoring, today announced it has received U.S. Food and Drug Administration (FDA) clearance for the ClearSight system.
To view the multimedia assets associated with this release, please click: http://www.multivu.com/players/English/7256451-edwards-fda-clearance-for-noninvasive-hemodynamic-monitoring-system/
The ClearSight system is a noninvasive monitor that provides clinicians access to valuable blood volume and blood flow information for patients at moderate or high risk of post-surgical complications, in whom invasive monitoring would not be used.
Using a cuff on the outside of the finger that is connected to the Edwards EV1000 clinical platform, the ClearSight system:
- utilizes Edwards' proven, gold-standard monitoring technologies that are used in hospitals around the world, and incorporates a finger cuff and software elements that have been used for the noninvasive monitoring of the blood pressure of astronauts in space;
- automatically provides up-to-the-minute information without inserting anything into the body as compared to the traditionally more invasive approach to monitoring, which today is only used in a fraction of those patients at risk of post-surgical complications; and thus
- provides an opportunity to extend the benefits of hemodynamic optimization, or proper fluid administration and balance within a patient's organs and tissues, to a broader patient population that could benefit from close monitoring, but may not receive it without a noninvasive option.
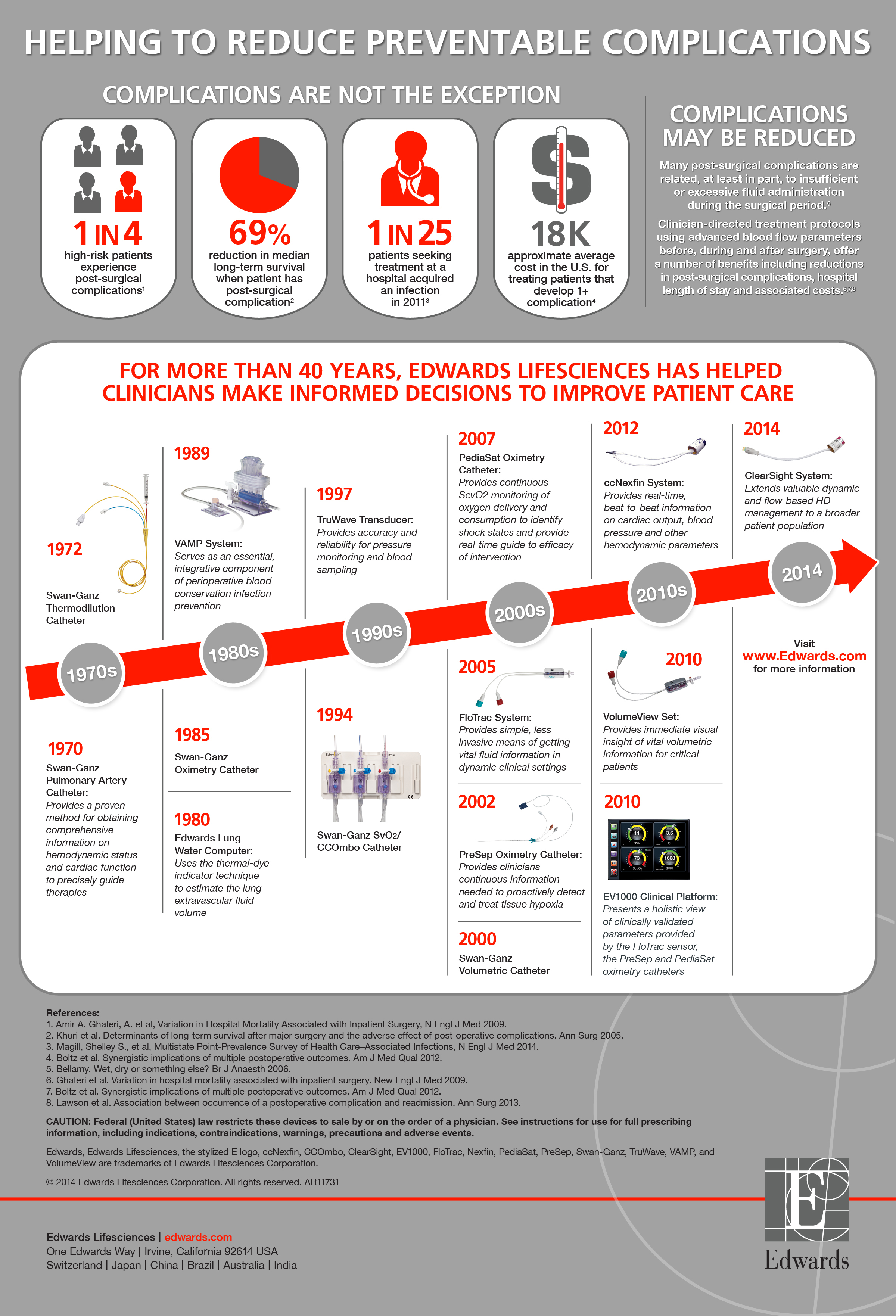
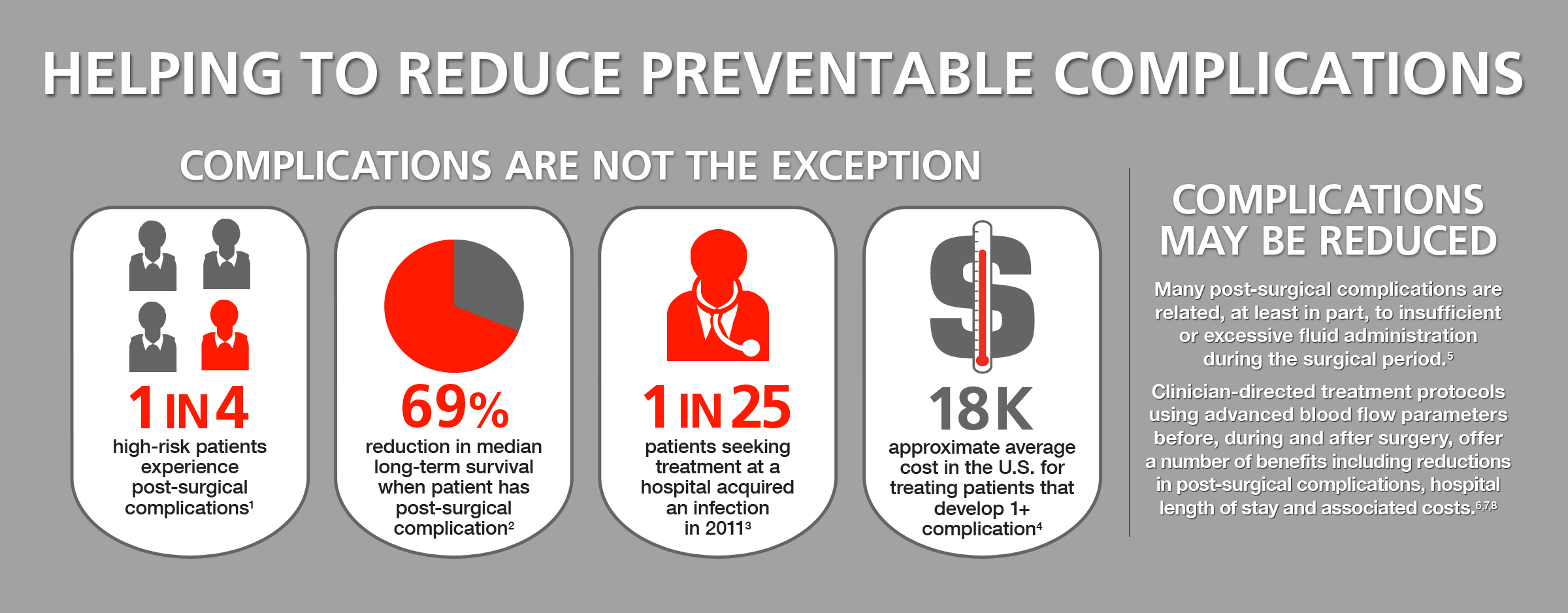
"Proper intraoperative management of moderate and high-risk surgery patients is critical to help reduce the risk of post-surgical complications," said Julie K. Thacker, M.D., surgical oncologist at Duke University Hospital. "Studies have indicated that patient outcomes are improved through monitoring and management of vital hemodynamic information through hemodynamic optimization protocols."
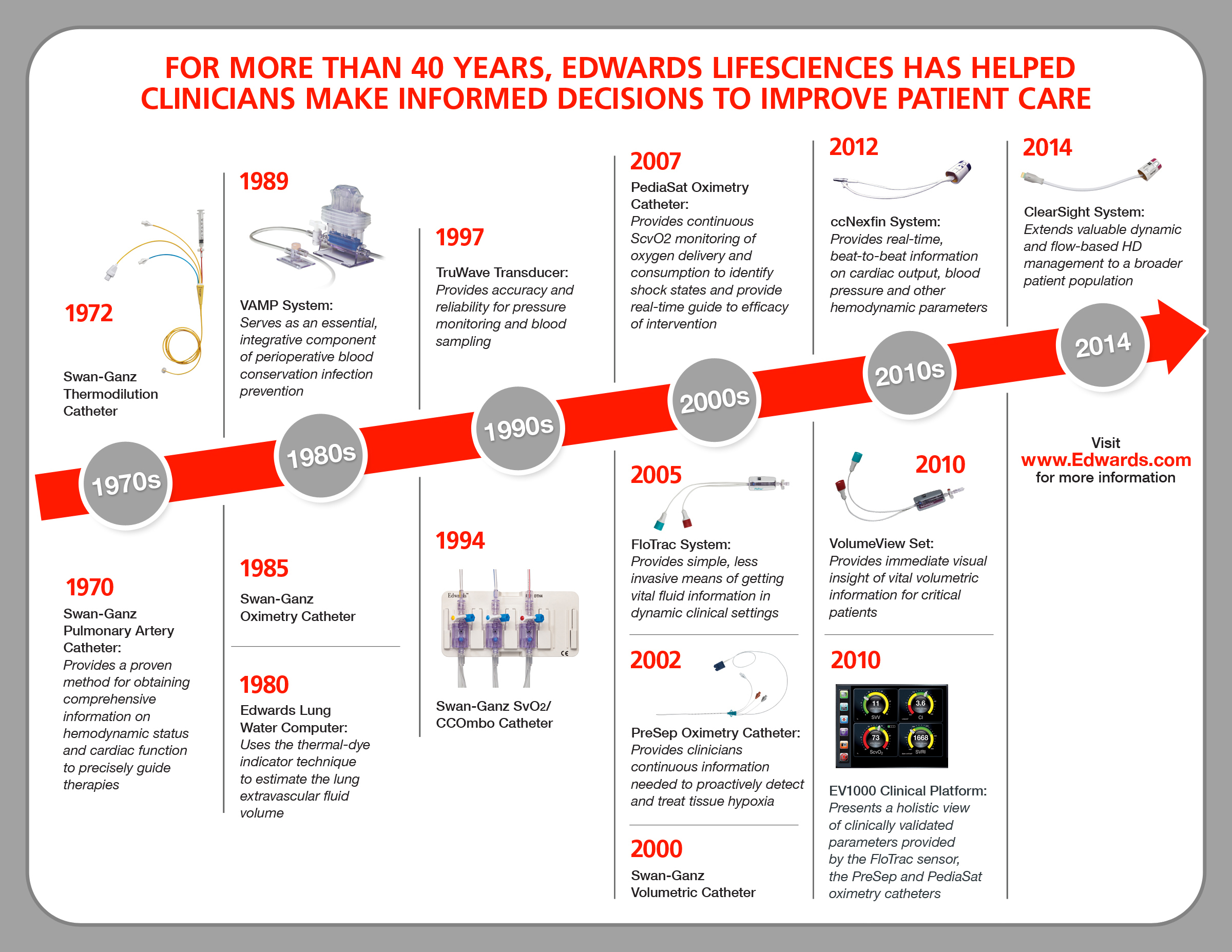
"The ClearSight system is the most advanced noninvasive monitor of its kind. This important development in the field of critical care medicine builds on Edwards' 40 years of experience in providing clinicians with tools to make more informed decisions that can advance patient care by helping to reduce complications, shorten hospital stays and lower associated costs," said Carlyn D. Solomon, Edwards' corporate vice president, critical care and vascular.
The ClearSight system is also available in CE Mark countries.
About Hemodynamic Monitoring
Hemodynamic monitoring is the measurement of blood circulation and cardiac function that allows clinicians to evaluate whether enough oxygen is being delivered to the organs and tissues. Healthcare providers use this information to detect changes or problems in a patient's health, which allows for more informed, immediate treatment decisions.
About Edwards Lifesciences
Edwards Lifesciences is the global leader in the science of heart valves and hemodynamic monitoring. Driven by a passion to help patients, the company partners with clinicians to develop innovative technologies in the areas of structural heart disease and critical care monitoring, enabling them to save and enhance lives. Additional company information can be found at www.edwards.com.
This news release includes forward-looking statements within the meaning of Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934. These forward-looking statements include, but are not limited to Mr. Solomon's and Dr. Thacker's statements and statements regarding design features, expected benefits and procedural outcomes with the ClearSight system. Forward-looking statements are based on estimates and assumptions made by management of the company and are believed to be reasonable, though they are inherently uncertain and difficult to predict.
Our forward-looking statements speak only as of the date on which they are made and we do not undertake any obligation to update any forward-looking statement to reflect events or circumstances after the date of the statement. Forward-looking statements involve risks and uncertainties that could cause actual results to differ materially from those expressed or implied by the forward-looking statements based on a number of factors including but not limited to expanded clinical experience, unexpected changes or delays related to product supply, quality and availability, changes in product indications or reimbursement levels, or regulatory decisions. These factors are detailed in the company's filings with the Securities and Exchange Commission including its Annual Report on Form 10-K for the year ended December 31, 2013.
Edwards, Edwards Lifesciences, the stylized E logo, ClearSight, and EV1000 are trademarks of Edwards Lifesciences Corporation. All other trademarks are the property of their respective owners.

To view the multimedia assets associated with this release, please click: http://www.multivu.com/players/English/7256451-edwards-fda-clearance-for-noninvasive-hemodynamic-monitoring-system/
SOURCE Edwards Lifesciences




