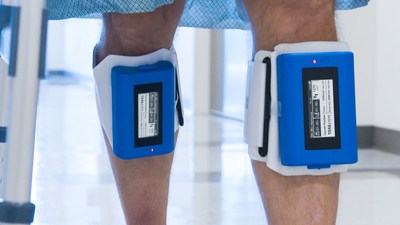
Recovery Force, LLC is pleased to announce the company has received U.S. Food and Drug Administration (FDA) 510(k) clearance for the use of the Movement and Compressions (MAC) System, a novel, data-driven, mobility measurement device designed for hospital and home use.
|
FISHERS, Ind., March 22, 2021 /PRNewswire/ -- Recovery Force, LLC is pleased to announce the company has received U.S. Food and Drug Administration (FDA) 510(k) clearance for the use of the Movement and Compressions (MAC) System, a novel, data-driven, mobility measurement device designed for hospital and home use. What It Is Why It Matters Studies show that patients often spend as much as 95% of their hospital stay in bed. Early patient mobility has been proven to combat non-reimbursable hospital-acquired events such as pneumonia (HAP), pressure ulcers, blood clots, and increased length-of-stay. The MAC System offers a solution that provides transparency and access to patient mobility data while seamlessly fitting into the complicated hospital workflow. "This is a significant innovation milestone for the healthcare industry," said Matthew Wyatt, President and CEO of Recovery Force. "The Movement and Compressions System integrates the physiological benefits of typical intermittent pneumatic compression (IPC) into a low-profile wearable device free of cords, hoses, and disruptive pump noise." Additionally, MAC embraces the continuum of care by providing at-risk patients discharged to the home setting access to the same mobility and compression data to actively engage and participate in their recovery. The Larger Trend Since 2018, Recovery Force has collaborated with Mayo Clinic to develop a unique mechanism for enhancing blood flow and optimizing clinical workflow. The company will commercially launch the MAC System in hospitals effective immediately, which will initiate the execution of a $1.8M NIH grant scheduled to kickoff at Eskenazi Hospital in Indianapolis, IN and Tufts Medical Center in Boston, MA. About Recovery Force Health (RF Health) Recovery Force Health is an all-encompassing digital health company focused on the development of data-driven solutions through wearable medical technology. From hospital to home, RF Health understands the ever-evolving nature of healthcare and how to best integrate innovation throughout the continuum of care. RF Health is a subsidiary of Recovery Force, LLC. To learn more please visit www.RFHealth.com. Contact Recovery Force Health Chief Commercialization Officer: Jason Bobay, jbobay@rfhealth.com Marketing Relations: Drew Martin dmartin@rfhealth.com Related: https://rfhealth.com/
SOURCE Recovery Force Health |