New Post-Hoc Analysis Presented at the Pediatric Academic Societies (PAS) Meeting
New Post-Hoc Analysis Presented at the Pediatric Academic Societies (PAS) Meeting
WARRINGTON, Pa., May 9, 2019 /PRNewswire/ -- Windtree Therapeutics, Inc. (OTCQB: WINT) today announced the results of a new post-hoc analysis of previously released phase 2 data that suggests AEROSURF® may reduce the overall incidence and severity of bronchopulmonary dysplasia (BPD) in premature infants with respiratory distress syndrome (RDS), regardless of whether or not the infant was ultimately intubated. The new data were recently presented at the Pediatric Academic Societies (PAS) Meeting, the leading event for academic pediatrics and child health research.
"As we continue to advance the AEROSURF program, data such as these play an important role in helping us formulate a better understanding of the possible benefits of AEROSURF beyond reducing the need for intubation in premature infants with RDS," said Steve Simonson, M.D., Senior Vice President and Chief Medical Officer at Windtree. "These data are consistent with previous analyses and suggest that, regardless of the need for intubation, early treatment with AEROSURF could potentially be an important factor in reducing the incidence and severity of BPD – a burdensome condition that can have a lasting impact on the patient and family and require substantial resources of the healthcare system."
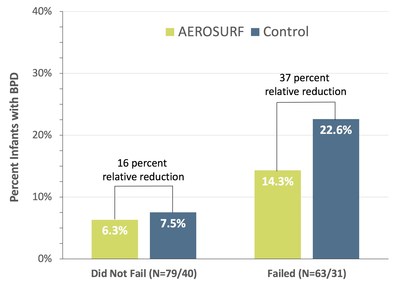
The new post-hoc analysis presented at PAS of pooled data from two studies, examining the presence of BPD in infants 28-32 weeks PMA who received any study treatment (Figure 1), suggested an advantage with AEROSURF treatment (versus control) with a relative reduction in BPD of 16 percent for infants that failed nCPAP (p=NS) and relative reduction of 37 percent for those that did not fail (p=NS). Although the differences were not statistically significant, these data suggest that in this population, AEROSURF may be able to reduce the overall incidence and severity of BPD, regardless of whether or not the infant was ultimately intubated. This exploratory result is encouraging and merits further study as we continue to advance the clinical development of AEROSURF.
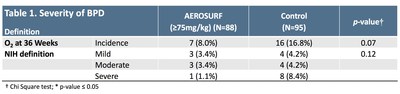
As presented in December 2018 at the Hot Topics in Neonatology annual meeting, an analysis of 261 premature infants (166 active, 95 control) showed a decrease in the incidence and severity of BPD in infants receiving doses of AEROSURF greater than or equal to 75 mg TPL/kg when compared to control in both trials (Table 1; p=0.02 for infants 26-to-28 weeks PMA, p=NS for infants 28-to-32 weeks PMA, and p=0.04 for all subjects).
About Bronchopulmonary Dysplasia
Bronchopulmonary dysplasia (BPD), a chronic lung disease of the newborn, is one of the most common complications of prematurity and treatments for RDS. It occurs in up to 40 percent of infants born at or before 28-week gestational age (GA) who have required intubation, mechanical ventilation and oxygen therapy. BPD is associated with ongoing pulmonary disease, neurodevelopmental impairment and increased healthcare utilization. BPD contributes to substantial patient morbidity and healthcare costs. Despite its importance, effective prevention and treatment strategies for BPD have been elusive and there is no approved treatment.
AEROSURF Phase 2 Clinical Program
The AEROSURF phase 2 clinical program in premature infants consisted of three multicenter, randomized, controlled clinical trials in a total of 341 premature infants receiving nasal continuous positive airway pressure (nCPAP) for RDS. The trials were designed to evaluate the safety, tolerability and potential effectiveness of aerosolized KL4 surfactant in reducing the need for invasive intubation and mechanical ventilation compared to infants receiving nCPAP alone (primary efficacy assessment). The diagnosis of BPD was a prespecified secondary endpoint. The data presented at PAS focused on a post-hoc, pooled analysis of two randomized, controlled phase 2 studies (NCT02528318 and NCT02636868) of premature infants who had not been previously intubated and, requiring supplemental oxygen (FiO2 ≥ 0.25), received the drug/device combination therapy AEROSURF with nCPAP or nCPAP alone (standard of care) at doses of 40 to 100 mg TPL/kg. Dosing occurred during the first day of life (median 5.1 hours from birth). An analysis of all subjects examined whether AEROSURF decreased BPD incidence and severity using 2 definitions of BPD (supplemental O2 at 36 weeks post-menstrual age (PMA) and NIH [Jobe 2001]).
Based on the results of AEROSURF phase 2 clinical program, the Company plans to conduct a 70-patient bridge study of its new phase 3 Aerosol Delivery System (ADS) medical device and work with regulators to develop a phase 3 regulatory and clinical plan. While the reduction of nCPAP failures and need for intubation is anticipated to remain the primary endpoint in the phase 3 study, measuring the potential impact on the incidence and severity of BPD will be an important secondary measure.
The AEROSURF phase 2 clinical program was supported, in part, by both a $1.9 million Phase II award under a $2.6 million Small Business Innovation Research (SBIR) grant and a $2.6 million Phase IIb grant from the National Heart, Lung, and Blood Institute of the National Institutes of Health (NIH) under parent award number R44HL107000. The content of this press release is solely the responsibility of the Company and does not necessarily represent the official views of the NIH.
About AEROSURF®
AEROSURF (lucinactant for inhalation) is a novel, investigational combination drug/device product that combines the Windtree's proprietary KL4 surfactant and aerosolization technologies. AEROSURF is being developed to potentially reduce or eliminate the need for endotracheal intubation and mechanical ventilation in the treatment of premature infants with respiratory distress syndrome (RDS). AEROSURF has received Fast Track designation from the Food and Drug Administration (FDA). The FDA's Fast Track program expedites the review of new drugs that treat serious or life-threatening conditions and address unmet medical needs.
About Windtree Therapeutics
Windtree Therapeutics, Inc. is a clinical-stage, biopharmaceutical and medical device company focused on the development of novel therapeutics intended to address significant unmet medical needs in important acute care markets. Windtree has four programs in clinical development and multiple pre-clinical programs that span respiratory and cardiovascular disease states. Three of Windtree's clinical programs are in late-stage development and include AEROSURF®, an innovative combination drug/device product candidate that is designed to deliver the Company's proprietary synthetic, peptide-containing surfactant non-invasively to premature infants with respiratory distress syndrome (RDS); istaroxime, a novel, dual-acting agent being developed to improve cardiac function in patients with acute heart failure while avoiding the unwanted side effects of existing treatments; and rostafuroxin, a novel precision drug product being developed to target hypertensive patients with certain genetic profiles in the important group of patients with resistant hypertension. Windtree also has multiple pre-clinical products including potential heart failure therapies delivered orally that are based on SERCA2a mechanism of action.
For more information, please visit the Company's website at www.windtreetx.com.
Forward-Looking Statements
To the extent that statements in this press release are not strictly historical, all such statements are forward-looking, and are made pursuant to the safe harbor provisions of the Private Securities Litigation Reform Act of 1995. These forward-looking statements are subject to certain risks and uncertainties that could cause actual results to differ materially from the statements made. Examples of such risks and uncertainties include those risks related to the post-merger integration of CVie Therapeutics Limited, including with respect to having two international locations; risks related to Windtree's ability to secure significant additional capital as and when needed, if at all; Windtree's product development programs, which are expected to involve time-consuming and expensive clinical trials that may be subject to potentially significant delays or regulatory holds, or fail; risks related to development of Windtree's aerosol delivery systems (ADS) and related components; risks related to the manufacture by contract manufacturers or suppliers of drug products, drug substances, ADS and other materials on a timely basis and in sufficient amounts; risks relating to rigorous regulatory requirements, including those of the U.S. Food and Drug Administration or other regulatory authorities that may require significant additional activities, or may not accept or may withhold or delay consideration of applications, or may not approve or may limit approval of Windtree's products; and other risks and uncertainties described in Windtree's filings with the Securities and Exchange Commission including the most recent reports on Forms 10-K, 10-Q and 8-K, and any amendments thereto.
![]() View original content to download multimedia:http://www.prnewswire.com/news-releases/new-phase-2b-analysis-suggests-aerosurf-may-reduce-incidence-and-severity-of-bronchopulmonary-dysplasia-in-preterm-infants-with-rds-300847795.html
View original content to download multimedia:http://www.prnewswire.com/news-releases/new-phase-2b-analysis-suggests-aerosurf-may-reduce-incidence-and-severity-of-bronchopulmonary-dysplasia-in-preterm-infants-with-rds-300847795.html
SOURCE Windtree Therapeutics, Inc.
Company Codes: NASDAQ-SMALL:WINT, OTC-PINK:WINT, OTC-QB:WINT




