Icosavax, Inc. (Nasdaq: ICVX), a biopharmaceutical company leveraging its innovative virus-like particle (VLP) platform technology to develop vaccines against infectious diseases, with an initial focus on life-threatening respiratory diseases and a vision of creating pan-respiratory vaccines for older adults, today provided a six-month immunogenicity update from its Phase 1/1b trial of IVX-121 against Respiratory Syncytial Virus (RSV).
- In new data from Phase 1/1b study, IVX-121 showed sustained immunologic response at six months; geometric mean titers (GMT) against RSV-A through day 180 persisting at 64-98% of the GMTs at day 28 in older adults -
- First clinical evidence of potential differentiation on durability with company’s VLP platform technology -
- IVX-121 continues to be generally well tolerated with no safety concerns observed in this six-month follow up -
- IVX-A12 (a bivalent of IVX-121 for RSV and IVX-241 for hMPV) progressing in Phase 1, and the only clinical-stage candidate targeting these two leading causes of pneumonia in one combination -
SEATTLE, Dec. 13, 2022 (GLOBE NEWSWIRE) -- Icosavax, Inc. (Nasdaq: ICVX), a biopharmaceutical company leveraging its innovative virus-like particle (VLP) platform technology to develop vaccines against infectious diseases, with an initial focus on life-threatening respiratory diseases and a vision of creating pan-respiratory vaccines for older adults, today provided a six-month immunogenicity update from its Phase 1/1b trial of IVX-121 against Respiratory Syncytial Virus (RSV). These new data demonstrate a sustained neutralizing antibody (nAb) response against RSV, lasting for at least six months after a single administration of IVX-121.
“We are delighted to share the six-month immunogenicity data from our Phase 1/1b trial of IVX-121. The potential for long-lasting immune responses was part of our founding hypothesis and preclinical rationale for developing combination VLP vaccines, so it is exciting to see this supportive initial clinical data on durability from our platform,” said Adam Simpson, Chief Executive Officer of Icosavax. “We continue to believe that there is a need for better vaccines, in particular the potential for combination respiratory vaccines with longer-lasting protection and low reactogenicity.”
IVX-121 (RSV) Phase 1/1b six-month immunogenicity update
IVX-121 continued to be generally well-tolerated with no safety concerns observed in this six-month follow up and no vaccine related serious adverse events (SAEs).
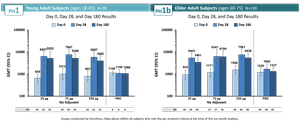
Data shown and described below illustrate the ongoing neutralizing antibody responses to a single administration of IVX-121 at three dosage levels (25, 75, 250 µg), in the groups without adjuvant. Samples were taken at baseline, day 28, and day 180, with neutralizing antibodies measured in international units (IU/mL) using the WHO international reference standard.
Figure 1 – RSV-A nAb, GMT expressed in IU/mL
In these day 180 immunogenicity data across IVX-121 unadjuvanted dosage groups, the pattern of durability was comparable in both young and older adult groups.
In the older adult portion of this study (Phase 1b), GMTs for RSV-A nAbs that were previously reported at up to 7,561 IU/mL at day 28 were observed to persist at up to 6,184 IU/mL through day 180. GMTs for RSV-A at day 180 were maintained within a range of 64-98% relative to the previously reported GMTs at day 28. GMTs for RSV-B showed greater variability but were maintained above baseline through day 180.
Icosavax plans to provide a 12-month immunogenicity update from an extension of this Phase 1b trial in mid-2023. The company also intends to present additional data from its Phase 1/1b clinical trial of IVX-121 at a future medical meeting.
About Icosavax
Icosavax is a biopharmaceutical company leveraging its innovative VLP platform technology to develop vaccines against infectious diseases, with an initial focus on life-threatening respiratory diseases and a vision for combination and pan-respiratory vaccines. Icosavax’s VLP platform technology is designed to enable multivalent, particle-based display of complex viral antigens, which it believes will induce broad, robust, and durable protection against the specific viruses targeted. Icosavax’s pipeline includes vaccine candidates targeting Respiratory Syncytial Virus (RSV) and human metapneumovirus (hMPV), as well as programs in severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and influenza. Icosavax was formed in 2017 to advance the breakthrough VLP technology from the Institute for Protein Design at the University of Washington with the goal to discover, develop, and commercialize vaccines against infectious diseases. Icosavax is located in Seattle.
For more information, visit www.icosavax.com.
Forward-Looking Statements
Statements contained in this press release regarding matters that are not historical facts are forward-looking statements. The forward-looking statements are based on the company’s current beliefs and expectations and include but are not limited to: the potential for the company’s VLP platform to result in safe and effective vaccines against infectious diseases, including IVX-121 for RSV and IVX-A12 for hMPV and RSV, and to be well suited for combination vaccines; and the company’s ability to advance its development programs and achieve the noted development milestones in 2023. Actual results may differ from those set forth in this press release due to the risks and uncertainties inherent in the company’s business, including, without limitation: the early stage of the company’s development efforts; the risk that results of a clinical trial at a particular time point may not predict final results and that an outcome may materially change as follow-up of subjects continues and following more comprehensive reviews of the data; the possibility of disappointing results in later clinical trials despite promising results in earlier preclinical research or clinical trials; potential unexpected adverse side effects or inadequate immunogenicity or efficacy of IVX-121 or IVX-A12 that may limit their development, regulatory approval, and/or commercialization; the company’s approach to the development of vaccine candidates, including its IVX-A12 combination bivalent RSV/hMPV VLP vaccine candidate, which is a novel and unproven approach; potential delays in the development process including without limitation in the enrollment, conduct of, and receipt of data from, clinical trials; the company’s dependence on third parties in connection with manufacturing, research, and clinical testing; the potential for challenges encountered in the manufacturing and scale up process; competing approaches limiting the commercial value of the company’s vaccine candidates; and other risks described in the company’s prior filings with the Securities and Exchange Commission (SEC), including under the heading “Risk Factors” in the company’s quarterly report on Form 10-Q for the quarter ended September 30, 2022 and any subsequent filings with the SEC. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof, and the company undertakes no obligation to update such statements to reflect events that occur or circumstances that exist after the date hereof. All forward-looking statements are qualified in their entirety by this cautionary statement, which is made under the safe harbor provisions of the Private Securities Litigation Reform Act of 1995.
Media Contact:
Jessica Yingling, Ph.D.
Little Dog Communications Inc.
jessica@litldog.com
858.344.8091
Investor Contact:
Laurence Watts
Gilmartin Group, LLC
laurence@gilmartinir.com
619.916.7620
A photo accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/b41b9280-8f6a-4825-bf77-2bcd9a3e519f