USGI Medical, Inc. (USGI) announced today the United States Food and Drug Administration (FDA) acknowledged the successful completion of a pilot study of its incisionless, endoscopic gastroplasty procedure, known as POSE2.0®, which evaluated weight loss in adults suffering from obesity (body mass index [BMI] 35-40 kg/m²) who have an obesity-related comorbidity, such as diabetes or hypertension.
- Company builds on its legacy of innovation in weight-loss surgery to make endoscopic gastroplasty an essential part of the primary obesity treatment toolkit -
- Lays foundation for pivotal study for a primary obesity indication in the U.S. -
SAN CLEMENTE, Calif., Dec. 12, 2023 (GLOBE NEWSWIRE) -- USGI Medical, Inc. (USGI), the pioneer of incisionless weight loss procedures and devices, announced today the United States Food and Drug Administration (FDA) acknowledged the successful completion of a pilot study of its incisionless, endoscopic gastroplasty procedure, known as POSE2.0®, which evaluated weight loss in adults suffering from obesity (body mass index [BMI] 35-40 kg/m²) who have an obesity-related comorbidity, such as diabetes or hypertension. Completing the pilot study lays the foundation for future regulatory submissions to enable marketing of POSE2.0® devices in the U.S.
The pilot study, which included four sites and 40 U.S. patients, was led by Barham Abu Dayyeh, MD, MPH, of Mayo Clinic (Rochester, Minn.), who served as Principal Investigator. Investigators evaluated patients’ safety, weight loss, and other effectiveness factors. Patients received lifestyle and nutritional care through the primary endpoint of one year.
Dr. Abu Dayyeh commented, “There is a growing body of global evidence that appears promising regarding the potential impact of this gastric remodeling procedure on obesity and related comorbidities."1
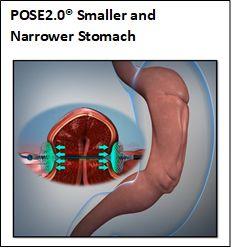
POSE2.0® uses USGI Medical’s Incisionless Operating Platform (IOP) to strategically place durable Snowshoe Suture Anchors in gastric tissue to both shorten and narrow the stomach. The procedure provides volumetric gastric restriction, like that achieved by a surgical sleeve gastrectomy, without surgically removing part of the stomach. Patients treated with the POSE2.0® procedure typically return to normal activities and lifestyles faster, compared to traditional laparoscopic and open abdominal surgery.
Mr. Arnold Podgorsky, CEO of USGI Medical, said, “As a pioneer in incisionless weight loss procedures, this acceptance marks an important milestone for USGI in validating the safety, durability and potential effectiveness of POSE2.0® devices for primary obesity. It also reflects our ongoing collaboration with leading bariatric specialists from around the world. We look forward to advancing a U.S. pivotal study for a primary obesity indication and continuing to build value for our shareholders.”
About Obesity
Obesity is an increasing, global public health issue. Patients with obesity are at major risk for developing a range of comorbid conditions, including cardiovascular disease (CVD), gastrointestinal disorders, type 2 diabetes (T2D), joint and muscular disorders, respiratory problems, and psychological issues, which may significantly affect their daily lives as well as increasing mortality risks. Obesity‐associated conditions are manifold; however, even modest weight reduction may enable patients to reduce their risk for CVD, diabetes, obstructive sleep apnea (OSA), and hypertension among many other comorbidities.2 A relatively small and simple reduction in weight, for example, of around 5%, can improve patient outcomes and may act as a catalyst for further change, with sustainable weight loss achieved through a series of incremental weight loss steps. According to the Center for Disease Control and Prevention (CDC), the prevalence of obesity among U.S. adults today is approximately 40%, a rate that has grown significantly over the preceding decades, putting financial strains on the U.S. healthcare system with annual medical costs totaling more than $150 billion in U.S. dollars.
About USGI Medical, Inc.
USGI Medical develops technologies to enable Incisionless Surgery – the treatment of diseases through the natural passageways of the body. USGI's Incisionless Operating Platform (IOP) provides physicians the operating platform and specialized tools they need to perform procedures through a patient's mouth or other natural orifices. USGI has demonstrated the capability to suture GI tract tissue reliably and durably without an incision, opening the way to a new generation of treatments for a wide variety of acute and chronic diseases. Operating through the body's natural orifices offers promise for less pain, shorter hospital stays, reduced risk of wound infection along with no external scarring from abdominal incisions – and is rapidly becoming an option demanded by patients and healthcare providers. USGI offers surgeons and gastroenterologists the tools they need to offer millions of potential patients a less invasive option to surgery. For more information, go to http://www.usgimedical.com/.
The Incisionless Operating Platform, including the g-Cath EZ Delivery Catheter with Snowshoe® Suture Anchors, has both CE Mark and US 510(k) Clearance for tissue approximation. The safety and effectiveness of the device has not been established in the United States so as to permit marketing for the treatment of obesity. The device is considered an investigational device in the United States and is thereby limited by Federal law to investigational use for obesity treatment.
For Further Information:
Media Contact:
Glenn Silver
Lazar-FINN Partners
glenn.silver@finnpartners.com
+1 646 871 8485
_______________________
1 Clin Gastroenterology Hepatology 2023 Jan; 21(1):81-89. e4, Endoscopy 2021 Nov; 53(11):1169-1173, Endoscopy 2023 Nov; 55(11):1028-1034, Am J Gastroenterology. 2023 Jun 1; 118(6):983-990; Gastrointestinal Endoscopy. 2022 Sep;96(3):479-486.
2 Cefalu, W. T. , Bray, G. A. , Home, P. D. , Garvey, W. T. , Klein, S. , Pi‐Sunyer, F. X. , … Ryan, D. H. , et al. (2015). Advances in the science, treatment, and prevention of the disease of obesity: Reflections from a diabetes care editors' expert forum. Diabetes Care, 38(8), 1567–1582
A photo accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/2f9f254f-581b-4f66-ae8e-b60d6c39c228