ST. PAUL, Minn., Jan. 5, 2016 /PRNewswire/ -- Torax Medical announced today that the FENIX® Continence Restoration System, an innovative approach to the treatment of fecal incontinence (FI), has received U.S. Food and Drug Administration (FDA) approval under a Humanitarian Device Exemption (HDE).
Experience the interactive Multimedia News Release here: http://www.multivu.com/players/English/7726351-torax-fenix-continence-restoration-system/
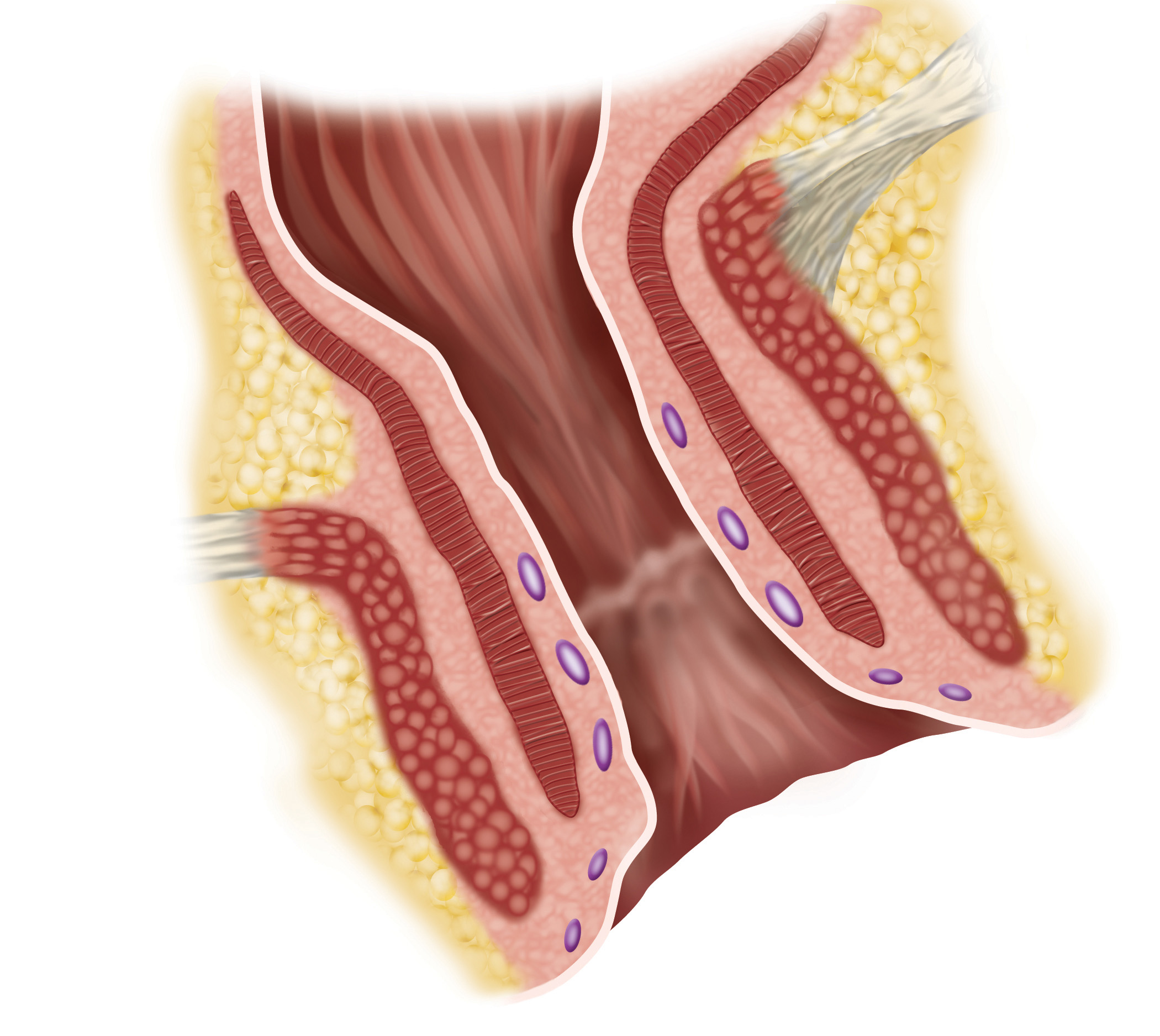
FI is the inability to control bowel movements due to a damaged or weak anal sphincter muscle. FI affects predominately women and is often an injury associated with childbirth. It is a condition that can occur at any age and is often devastating to a person's quality of life. For older people, fecal incontinence often results in early institutionalization because family members have difficulty coping with the problem at home.
Todd Berg, President and CEO for Torax Medical said, "The FENIX Continence Restoration System represents a vital treatment option for patients suffering from this debilitating problem. The FENIX device builds on our Magnetic Sphincter Augmentation (MSA) technology that has been proven to be effective in treating gastro-esophageal reflux disease (GERD) with our LINX Reflux Management System. The FENIX device has been successively marketed in Europe since 2011; we are excited to now have this procedure available for patients in the U.S."
"The FENIX device is a welcome addition to the limited number of treatment options currently available for patients suffering from fecal incontinence. This treatment option preserves the native anatomy and can offer the patient more control and improved quality of life. The FENIX device begins working immediately and importantly does not require any patient interaction or adjustments by a physician," said Anders Mellgren, MD, PhD, FACS, FASCRS, Professor, Chief Division of Colon & Rectal Surgery, Department of Surgery, University of Illinois at Chicago.
The Disease
Fecal Incontinence affects an estimated 30 million people in the U.S. and Europe alone. FI results from damaged or weakening anal sphincter muscle. The disease primarily affects women, but men can also develop FI. The impact of FI on patients' quality of life is often debilitating, causing absence from work, constant risk of embarrassment, and the inability to engage in routine activities. Treatment options are very limited with many patients ultimately requiring a colostomy.
The FENIX Continence Restoration System
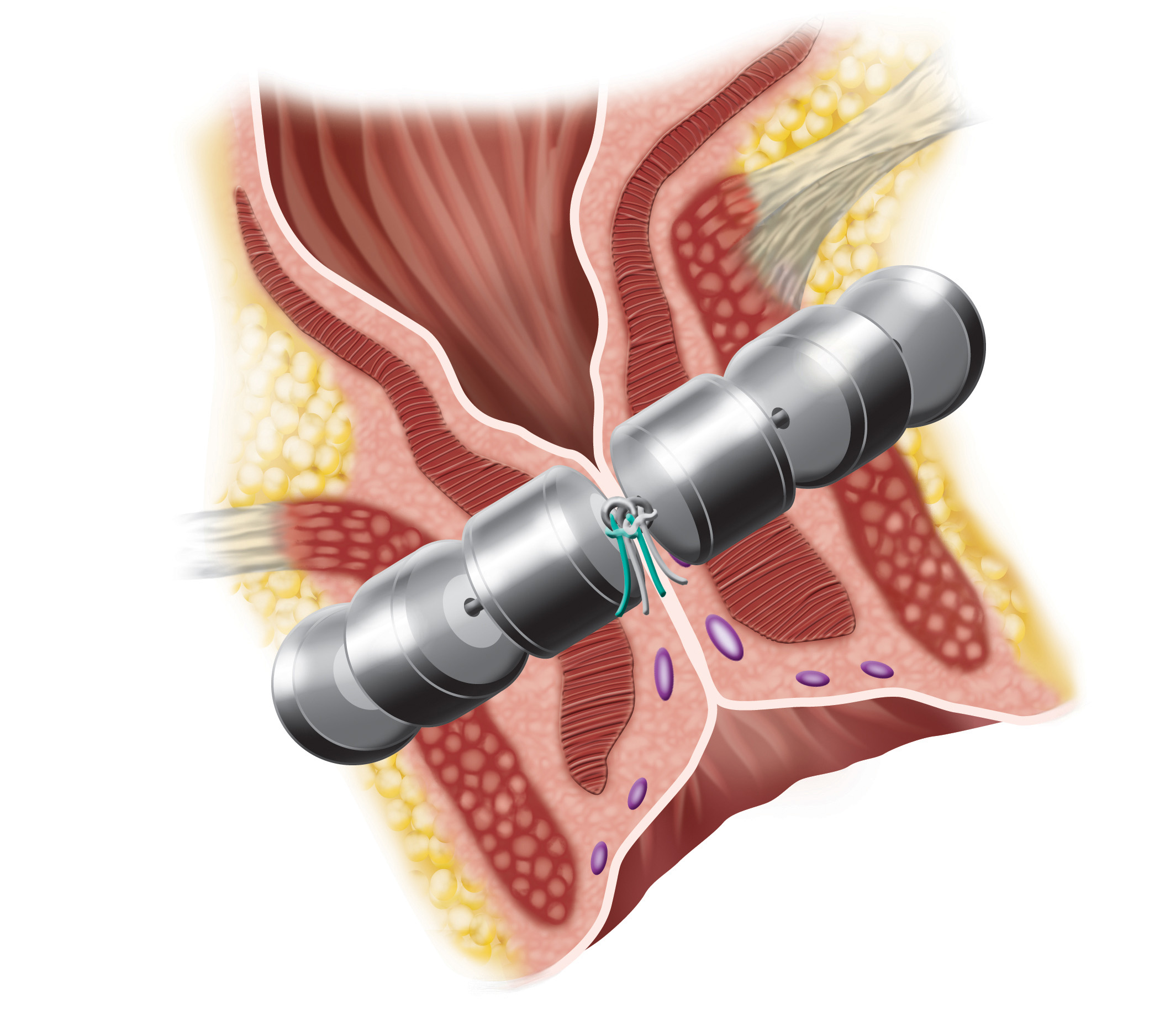
The FENIX Continence Restoration System is designed to treat fecal incontinence by augmenting the incompetent anal sphincter muscle. The FENIX System is a small, flexible band of interlinked titanium beads with magnetic cores. The magnetic attraction between the beads is temporarily released to allow for the intentional passage of stool. The FENIX System begins working immediately after implant and does not require activation by the patient or post-operative adjustments by a physician. FENIX does require a surgical procedure and is associated with potential risks and contraindications. For more information on FENIX, please visit www.toraxmedical.com.
About HDEs
Humanitarian Use Devices (HUDs) facilitate the development of medical devices intended to treat or diagnose a disease or condition affecting fewer than 4,000 people in the United States every year. To receive approval of a "Humanitarian Device Exemption" (HDE) application, a company must demonstrate the product's safety and probable benefit in lieu of a product's safety and effectiveness. For any center in the United States to be able to offer this treatment option for fecal incontinence (Torax Medical's FENIX Continence Restoration System), approval is required from an Institutional Review Board (IRB), a committee that approves, monitors, and reviews research within that center.
About Torax Medical
Torax Medical, Inc. is a privately-held medical device company headquartered in St. Paul, Minnesota that develops and markets products designed to treat sphincter disorders utilizing its technology platform, Magnetic Sphincter Augmentation (MSA). Torax Medical is currently marketing the LINX® Reflux Management System for the treatment of GERD and the FENIX® Continence Restoration System for the treatment of Fecal Incontinence (FI) in the U.S. and Europe. For more information, please visit www.toraxmedical.com.
Maggie Wallner
Torax Medical, Inc.
mwallner@toraxmedical.com
651-361-8900
To view the original version on PR Newswire, visit:http://www.prnewswire.com/news-releases/torax-medical-receives-fda-approval-for-its-fenix-continence-restoration-system-to-treat-fecal-incontinence-300199466.html
SOURCE Torax Medical Inc.





