Are patients being adequately protected when they are involved in studies of new medical therapies and devices?
CHICAGO, May 1, 2019 /PRNewswire/ -- Are patients being adequately protected when they are involved in studies of new medical therapies and devices?
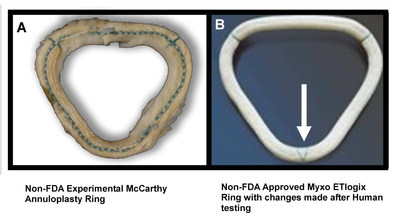
That question is at the heart of a story published Sunday in the Wall Street Journal, as well as an upcoming hearing (May 2) in a Chicago courtroom.
What should the rules and protocols be for medical studies and reviews? Should patients have to give their consent? Should doctors and other experts who question the safety of studies be freely allowed to voice their concerns?
The Federal Laws in the United States and globally are clear for human experimentation, full informed consent and oversight to avoid harm during drug and device testing.
The Thursday hearing involves a cardiac surgeon who implanted a non-FDA-approved heart valve ring he invented into hundreds of patients. At issue is whether he was simply recording outcomes after standard procedures or conducting a human research study and/or implanting investigational devices without patients' consent.
Dr. Nalini Rajamannan, a cardiologist who initially was involved in those studies, realized the patients had not given consent and provided years' worth of documents related to the Model 5100 testing to the US Department of Health and Human Services.
The Wall Street Journal exposes similar patient protection issues in a separate clinical trial regarding new approaches to treating sepsis. In an article published on April 28, 2019, Tom Burton writes of the internal conflicts over patients' protections in the National Institutes of Health-funded Clinical Trial CLOVERS (NCT03434028).
HHS leaders, Dr. Lawrence Tabak and Dr. Francis Collins have prohibited NIH expert scientists from being interviewed by the Office for Human Research Protections (OHRP), a federal agency mandated with assuring American patients aren't harmed in clinical research.
Dr. Michael Carome of the nonpartisan consumer group Public Citizen recommends ceasing the CLOVERS TRIAL until the issues are resolved. The day after the original WSJ story, a follow up reported that Public Citizen and the HHS Inspector General were already seeking answers to the questions raised by scientists regarding the CLOVERS TRIAL.
In the heart valve case, several patients interviewed by the Chicago Tribune regarding the clinical trial testing heart devices said they had never given consent prior to their surgeries.
For years, HHS officials have failed to inform the patients, despite head of the device branch, Dr. Shuren, confirming to the Chicago Tribune that the model 5100 was experimental during the clinical testing.
Dr. Rajamannan contacted the NIH/FDA/OHRP leaders from 2008-2019, to report to HHS that the patients did not receive informed consent. The agencies all failed to inform the patients of the terminated federal clinical trial what had happened, as is revealed in the recently published The Myxo Report.
The FDA released a preliminary report on their investigation to Senator Grassley(IA) and the late Senator Richard Lugar,(IN) but failed to report all of the adverse events and the heart attack suffered by patient Maureen Obermeier, who is at the center of Thursday's hearing.
US Senate Finance and Judiciary Investigation and records discovered by Iowa Senator Charles Grassley and reported by the Daily Northwestern, revealed possible issues with the angle of the device. It may not have been obvious to the surgeons which side was front or back when they placed the device in patients' hearts. This discovery led to major modifications of the device after the initial testing was completed, including a green mark to indicate the front from the back.
Model 5100 has been recalled and now is off the market around the world, but HHS leaders and Northwestern University still have not informed the patients of the clinical testing of these details regarding the experimental heart valve. Sen. Ron Johnson from Wisconsin contacted the HHS inspector general regarding this issue and is awaiting a response, and Congressman Glenn Grothman(WI) contacted the FDA regarding this issue and is awaiting a response also.
Patient rights are at issue in both the sepsis case outlined by WSJ and the heart valve case being heard Thursday in a Chicago courtroom.
Documents recently released under a Freedom of Information Act request prompted heart valve patient Maureen Obermeier to seek a new trial. A hearing will take place on May 2, 2019, in Cook County Circuit Court regarding the case, 08-L-012426 Obermeier versus Northwestern Memorial Hospital. The hearing in Judge James McGing's courtroom 2505, Daley Center at 9:30 am, will discuss the status of Petition 2-1401, involving fraudulent concealment of information as related to the failure to inform patients of the experimental nature of the device and the termination of the study by Northwestern University.
Link to FDA FOIA Documents:
https://www.prnewswire.com/news-releases/foia-documents-cardiac-surgeon-implanted-experimental-devices-without-consent-300836043.html
Dr. Nalini Rajamannan is a heart valve expert in the field of cardiovascular medicine. She has been researching heart valve disease for 31 years. She earned her undergraduate science pre-professional degree from the University of Notre Dame, her Medical Doctorate from Mayo Medical School and her post-graduate training in Internal Medicine and Cardiology at the Mayo Clinic. She also worked at the Mayo Clinic as a staff consultant in Internal Medicine. Currently, she practices consultative medicine specializing in Cardiac Valvular Heart Disease at Most Sacred Heart of Jesus Cardiology and Valvular Institute, WI.
SOURCE Dr. Nalini Rajamannan





