Theralase® Technologies Inc. (“Theralase” or the “Company”) (TSXV:TLT)(OTCQB:TLTFF), a clinical stage pharmaceutical company dedicated to the research and development of light activated Photo Dynamic Compounds (“PDCs”) and their associated drug formulations intended to safely and effectively destroy various cancers, bacteria and viruses released its unaudited interim consolidated condensed 2Q2020 financial statements.
TORONTO, ON / ACCESSWIRE / August 28, 2020 / Theralase® Technologies Inc. ("Theralase" or the "Company") (TSXV:TLT)(OTCQB:TLTFF), a clinical stage pharmaceutical company dedicated to the research and development of light activated Photo Dynamic Compounds ("PDCs") and their associated drug formulations intended to safely and effectively destroy various cancers, bacteria and viruses released its unaudited interim consolidated condensed 2Q2020 financial statements.
Financial Highlights:
Total revenue for the six-month period ended June 30, 2020, decreased to $293,453 from $370,436 for the same period in 2019, a 21% decrease.
In Canada, revenue decreased 23% to $255,146 in 2020 from $333,047 in 2019. In the US, revenue decreased 49% to 12,267 in 2020 from $23,926 in 2019. International revenue increased 93% to $26,041 for 2020 from $13,463 in 2019. The decrease in total revenue in 2020 is primarily attributed to the COVID-19 pandemic as a majority of health care practitioners elected to temporarily close their practices and place any purchasing decisions on temporary or permanent hold
Cost of sales for the six-month period ended June 30, 2020 was $230,095 which included a one-time provision for inventory of $77,075 resulting in an adjusted cost of sales of $153,020 or 52% of revenue with an adjusted gross margin of $140,433 or 48% of revenue, compared to a cost of sales of $267,644 or 72% of revenue in 2019, resulting in a gross margin of $102,792 or 28% of revenue. Cost of sales is represented by the following costs: raw materials, subcontracting, direct and indirect labour and the applicable share of manufacturing overhead. The gross margin decrease, as a percentage of sales, year over year, is attributed to decreased sales and fixed production salaries for the TLC-1000 and TLC-2000 product lines.
Selling expenses for the six-month period ended June 30, 2020 decreased to $229,998, from $344,501 in 2019, a 33% decrease. The decrease in selling expenses is primarily due to the restructuring of the Canadian and US sales and marketing departments, resulting in the resignation and/or termination of certain sales and marketing personnel and reduced travel expenditures due to the COVID-19 pandemic.
Administrative expenses for the six-month period ended June 30, 2020 decreased to $965,824 from $1,062,087 in 2019, a 9% decrease. The decrease in administrative expenses is primarily attributed to decreased spending on general and administrative expenses (48%) and administrative salaries (58%) due to the restructuring of the administrative department, resulting in the termination of certain administrative personnel.
Net research and development expenses for the six-month period ended June 30, 2020 increased to $2,219,057 from $1,303,375 in 2019, a 70% increase. The increase in research and development expense are primarily due to increased expenses for operating the Phase II Non-Muscle Invasive Bladder Cancer ("NMIBC") Clinical Study ("Study II"). Research and development expenses represented 67% of the Company's operating expenses for the six-month period ended June 30, 2020 and represent investment into the research and development of the Company's PDT technology.
The net loss for the six-month period ended June 30, 2020 was $3,267,624 which included $642,709 of net non-cash expenses (i.e.: amortization, stock-based compensation expense and foreign exchange gain/loss). This compared to a net loss for the same period in 2019 of $2,612,268 which included $194,361 of net non-cash expenses.
The PDT division represented $2,385,877 of this loss (73%) for the six-month period ended June 30, 2020.
The increase in net loss is primarily attributed to the following:
- Increased investment in the clinical expense of Study II.
- Decreased sales of the TLC-1000 and TLC-2000 due to market uncertainty directly attributable to the COVID-19 pandemic.
Operational Highlights:
COVID-19 Update: Theralase® continues to experience reduced sales due to the ongoing COVID-19 pandemic and has taken actions to reduce expenses by eliminating non-essential personnel and imposing a temporary hiring freeze, to be lifted, subject to the Canadian and United States economies demonstrating recovery from COVID-19.
Clinical Study Site Status: Theralase® has successfully launched 4 Canadian study sites with 1 additional Canadian clinical study site in advanced negotiation.
Three out of four Canadian clinical study sites have re-commenced new patient enrollment and treatment in Study II, specifically:

MUHC currently remains closed due to COVID-19, for new patient enrollment and treatment; however, patients who have already been enrolled and treated and who are eligible for second treatment will receive their second treatment.
Theralase® is in advanced discussions to launch a number of US based clinical study sites later in the year, subject to the United States economy recovering from the COVID-19 pandemic. The US based Trial Management Organization ("TMO") could potentially launch 4 clinical study sites in 4Q2020 and commence Study II patient enrollment and treatment as early as 1Q2021.
Study II Interim Data: Study II enrolled and treated 12 patients, with the following results:
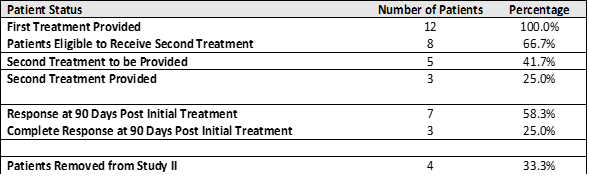
Of the 7 patients, who demonstrated a Response to the Study Treatment defined as negative cystoscopy (no evidence of cancer in their bladders) or negative urine cytology (no evidence of the urothelial carcinoma cells in their urine)) at 90 days post initial treatment:
- 43% achieved a Complete Response ("CR") (negative cystoscopy and negative urine cytology)
- 43% achieved a Response (negative cystoscopy and positive or suspicious cytology)
- 14% achieved a Response (suspicious cystoscopy and negative cytology)
Additional Oncology Targets. The Company has demonstrated significant anti-cancer efficacy of Rutherrin® (patented formulation of the Company's lead PDC (TLD-1433) and transferrin), when activated by laser light or radiation treatment across numerous preclinical models; including: Glio Blastoma Multiforme ("GBM") and Non-Small Cell Lung Cancer ("NSCLC").
The Company is planning to commence toxicology studies with Rutherrin® to determine the maximum recommended human dose of the drug, when administered systemically into the human body, via intravenous injections. Due to the limitations of using laser light to activate Rutherrin® in deep oncological targets, Theralase's research strongly suggests that Rutherrin® may be activated with radiation therapy, which is able to increase the ‘tumour's damage zone' and the effectiveness of the anti-cancer therapy beyond the reach of light in the body.
Additional Virus Targets. Theralase executed a Sponsored Research Agreement ("SRA") with the University of Manitoba ("UM") Medical Microbiology department to commence development of a coronavirus vaccine and therapy utilizing Theralase's patented and proprietary PDCs. According to the SRA, UM will conduct experiments in conjunction with Theralase for the research and development of a coronavirus vaccine and therapeutic to be further evaluated in animal then human clinical testing in 2021.
The primary objective of the SRA executed between the UM and Theralase® is to investigate the ability of Theralase's® lead PDC in the destruction of a variety of viruses; including: H1N1 Influenza, Zika, coronaviruses and of course COVID-19. The secondary objective is to optimize the concentration of PDC required, the activation methodology and how to potentially administer the treatment to humans to be used as a vaccine (prevention of a patient from contracting COVID-19) and as a therapeutic (treatment of a patient who has already contracted COVID-19). The research is primarily directed to in-vitro (Petri dish of viruses) analysis, but based on these initial experiments, Theralase hopes to expand the work, in conjunction with Dr. Coombs, to in-vivo (small animal) analysis, toxicology (optimized doses for human delivery) and finally human testing through Phase I (safety), Phase II (efficacy) and eventually Phase III (efficacy in a larger population) clinical studies. If successful through a Phase III clinical study, and with the successful regulatory approval of Health Canada, the technology could be commercialized across Canada for the benefit of all Canadians.*
* The Company does not claim or profess that they have the ability to treat, cure or prevent the contraction of the COVID-19 Coronavirus.
About Study II
Study II utilizes the Therapeutic Dose (0.70 mg/cm2) of TLD-1433 and is focused on the enrollment and treatment of approximately 100 Bacillus Calmete Guérin ("BCG")-Unresponsive NMIBC patients presenting with Carcinoma In-Situ ("CIS") in approximately 20 clinical study sites located in Canada and the US.
Study II has a:
- Primary endpoint of efficacy (defined by Complete Response ("CR")) at any point in time
- Secondary endpoint of duration of CR at 360 days post-initial CR (approximately 450 days post initial Study treatment)
- Tertiary endpoint of safety measured by incidence and severity of Adverse Events ("AEs") grade 4 or higher that do not resolve within 450 days post-initial treatment
"For single-arm trials of patients with BCG-unresponsive disease, the FDA defines CR as at least one of the following:
- Negative cystoscopy and negative (including atypical) urine cytology
- Positive cystoscopy with biopsy-proven benign or low-grade NMIBC and negative cytology
- For intravesical therapies without systemic toxicity, the FDA includes, in the definition of a CR, negative cystoscopy with malignant urine cytology, if cancer is found in the upper tract or prostatic urethra and random bladder biopsies are negative.
Intravesical instillation does not deliver the investigational drug to the upper tract or prostatic urethra; therefore, the development of disease in these areas cannot be attributed to a lack of activity of the investigational drug. Thus, sponsors can consider patients with new malignant lesions of the upper tract or prostatic urethra, who have received intravesical therapy to have achieved a CR in the primary analysis; however, sponsors should record these lesions and conduct sensitivity analyses in which these patients are not considered to have achieved a CR."1
About Theralase® Technologies Inc.
Theralase® is a clinical stage pharmaceutical company dedicated to the research and development of light activated Photo Dynamic Compounds and their associated drug formulations intended to safely and effectively destroy various cancers, bacteria and viruses.
Additional information is available at www.theralase.com and www.sedar.com
This news release contains "forward-looking statements" which reflect the current expectations of the Company's management for future growth, results of operations, performance and business prospects and opportunities. Such statements include, but are not limited to, statements regarding the Company's proposed development plans with respect to Photo Dynamic Compounds and their drug formulations. Wherever possible, words such as "may", "would", "could", "should", "will", "anticipate", "believe", "plan", "expect", "intend", "estimate", "potential for" and similar expressions have been used to identify these forward-looking statements. These statements reflect management's current beliefs with respect to future events and are based on information currently available to management. Forward-looking statements involve significant risks, uncertainties and assumptions including with respect to the ability of the Company to: adequately fund, secure the requisite regulatory approvals to commence and successfully complete a Phase II NMIBC clinical study in a timely fashion and implement its development plans. Many factors could cause the Company's actual results, performance or achievements to be materially different from any future results, performance or achievements that may be expressed or implied by such forward-looking statements; including, without limitation, those listed in the filings made by the Company with the Canadian securities regulatory authorities (which may be viewed at www.sedar.com). Should one or more of these risks or uncertainties materialize or should assumptions underlying the forward looking statements prove incorrect, actual results, performance or achievements may vary materially from those expressed or implied by the forward-looking statements contained in this news release. These factors should be considered carefully and prospective investors should not place undue reliance on the forward-looking statements. Although the forward-looking statements contained in the press release are based upon what management currently believes to be reasonable assumptions, the Company cannot assure prospective investors that actual results, performance or achievements will be consistent with these forward-looking statements. The Company disclaims any intention or obligation to revise forward-looking statements whether as a result of new information, future developments or otherwise except as required by law. All forward-looking statements are expressly qualified in their entirety by this cautionary statement.
Neither TSX Venture Exchange nor its Regulation Services Provider (as that term is defined in the policies of the TSX Venture Exchanges) accepts responsibility for the adequacy or accuracy of this release.
For More Information:
1.866.THE.LASE (843-5273) x 224
416.699.LASE (5273) x 224
Kristina Hachey CPA, Chief Financial Officer
khachey@theralase.com
www.theralase.com
1 "BCG-Unresponsive Nonmuscle Invasive Bladder Cancer: Developing Drugs and Biologics for Treatment - Guidance for Industry" Dated: February 2018
SOURCE: Theralase® Technologies Inc.
View source version on accesswire.com:
https://www.accesswire.com/603871/Theralase-Release-2Q2020-Financial-Statements




