TUCSON, AZ--(Marketwire - September 14, 2011) -
| Highlighted Links |
Pastor Returns to Pulpit |
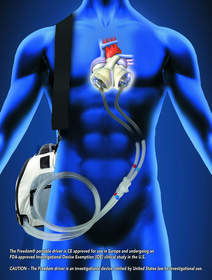
Freedom Portable Driver |
"Through the peer-nomination and peer-review process, we uncover the best of the best of the best in science and technology," says James P. Clark, Founder and Chairman of WTN. "We discover whose work is going to have the greatest likely impact over time. The World Technology Awards give a snapshot of the amazing science and technology revolutions in process."
SynCardia is currently conducting an FDA-approved Investigational Device Exemption (IDE) clinical study of its Freedom® portable driver, the first wearable power supply for the SynCardia temporary Total Artificial Heart. For the first time in U.S. history, stable patients who meet study criteria are leaving the hospital without human hearts to wait for a matching donor heart at home. Prior to the development of the Freedom driver, U.S. Total Artificial Heart patients were confined to the hospital for months, sometimes years while they waited for a transplant because the only FDA-approved driver, "Big Blue," is hospital-based and weighs 418 pounds.
The other 5 finalists in the Health & Medicine category include:
- Bio-Path Holdings - developed drug delivery technology that provides systemic distribution of nucleic acid drugs through simple intravenous transfusion
- Institute of Microelectronics, Singapore - developed a real-time multifunctional integrated electrocardiogram (ECG) signal processing scheme
- NeuroPace - developed technology designed to decrease or eliminate epilepsy episodes through an implant which monitors the patient's brain, and delivers electric signals to prevent seizures by disrupting any abnormal activity
- Second Sight Medical Products - created a retinal prosthesis to provide sight to patients blinded from outer retinal degenerations
- SpectraScience - developed a technology platform to instantly determine if tissue is normal, pre-cancer or cancerous, without the need for exploratory biopsy.
The winners of the World Technology Awards will be announced during a ceremony at the United Nations on October 26, at the close of the two-day World Technology Summit.
CAUTION - The Freedom® portable driver is an investigational device, limited by United States law to investigational use.
About the SynCardia temporary Total Artificial Heart
SynCardia Systems, Inc. (Tucson, AZ) is the privately-held manufacturer of the world's first and only FDA, Health Canada and CE approved Total Artificial Heart. Originally used as a permanent replacement heart, the Total is currently approved as a bridge to transplant for people dying from end-stage biventricular heart failure. More than 950 implants account for more than 230 patient years of life.
Similar to a heart transplant, SynCardia's Total Artificial Heart replaces both failing heart ventricles and the four heart valves. It is the only device that eliminates the symptoms and source of end-stage biventricular failure. The Total Artificial Heart provides immediate, safe blood flow of up to 9.5 liters per minute through both ventricles. This high volume of safe blood flow helps speed the recovery of vital organs, helping make the patient a better transplant candidate.
In March 2011, Fast Company magazine ranked SynCardia #20 among the World's 50 Most Innovative Companies "for giving mobility to artificial heart recipients." Also in March, the longest running health and wellness series on public television, "Healthy Body, Healthy Mind," produced a 30-minute program featuring SynCardia's Total Artificial Heart. View here
For additional information, please visit: http://www.syncardia.com
Like SynCardia on Facebook
Follow SynCardia on Twitter - @SynCardia
Connect with SynCardia on LinkedIn
Media Contact:
Don Isaacs
Vice President of Communications
SynCardia Systems, Inc.
Cell: (520) 955-0660
Email Contact


 Digg this
Digg this Bookmark with del.icio.us
Bookmark with del.icio.us Add to Newsvine
Add to Newsvine


