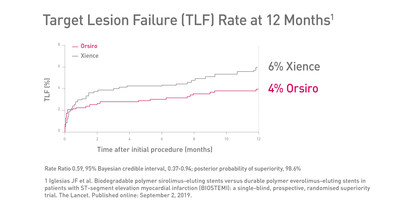
BIOTRONIK’s ultrathin Orsiro® stent demonstrated superiority over Xience with respect to target lesion failure (TLF) at 12 months, according to newly released data from the BIOSTEMI trial
|
PARIS and LAKE OSWEGO, Ore., Sept. 3, 2019 /PRNewswire/ -- BIOTRONIK's ultrathin Orsiro® stent demonstrated superiority over Xience with respect to target lesion failure (TLF) at 12 months, according to newly released data from the BIOSTEMI trial.1 Yesterday at the European Society of Cardiology 2019 Congress, Dr. Juan Fernando Iglesias, Geneva University Hospitals, Geneva, Switzerland unveiled the results of the randomized controlled trial (RCT) in a late-breaking session. The results have also been published in The Lancet.2
BIOSTEMI is the first direct comparison between two newer-generation drug-eluting stents (DES) in patients presenting with acute ST-segment elevation myocardial infarction (STEMI). BIOSTEMI is also the first RCT to demonstrate superiority between two contemporary DES. "The BIOSTEMI trial proves what the BIOSCIENCE STEMI subgroup analysis had already suggested: Different platform designs can really make a difference," explained Principal Investigator, Dr. Iglesias. "Based on the data, compared to the Xience stent, Orsiro is a superior solution for STEMI patients. In this patient group, vascular healing is a challenge due to the complex pro-thrombotic and inflammatory milieu. With Orsiro, we can incrementally improve their care path." Caused by a complete thrombotic occlusion in a coronary vessel, STEMI is the most acute manifestation of coronary artery disease, with substantial rates of morbidity and mortality.3 STEMI patients represent about 30% of all primary percutaneous coronary intervention (PCI) cases.4 BIOSTEMI is an investigator-initiated, multicenter, superiority trial using a Bayesian design to compare biodegradable polymer sirolimus-eluting stents to durable polymer everolimus-eluting stents in 1,300 patients with acute myocardial infarction. Orsiro demonstrated superiority in the clinical primary endpoint of TLF5 with an incidence of 4%, in comparison to Xience, with an incidence of 6% at 12 months (Rate Ratio 0.59, 95% Bayesian credible interval, 0.37-0.94; posterior probability of superiority, 98.6%). The difference in TLF was driven by lower rates of clinically-indicated target lesion revascularization in patients treated with ultrathin Orsiro compared to Xience. "At BIOTRONIK we always aim to advance the standard of care," commented Dr. Alexander Uhl, Senior Vice President Corporate Marketing at BIOTRONIK. "The BIOSTEMI results are groundbreaking. Our data demonstrates a 41%6 lower risk for TLF with Orsiro, delivering a significant benefit for interventionalists and their STEMI patients." References Orsiro DES is not currently indicated for STEMI patients. Orsiro is indicated for improving coronary luminal diameter in patients, including those with diabetes mellitus, with symptomatic heart disease, stable angina, unstable angina, non-ST elevation myocardial infarction or documented silent ischemia due to atherosclerotic lesions in the native coronary arteries with a reference vessel diameter of 2.25 mm to 4.0 mm and a lesion length of ≤ 36 mm. About BIOTRONIK For more information, visit: www.orsiro.com
SOURCE BIOTRONIK |