Stratasys PolyJet technology and Materialise FDA-cleared software now most versatile 3D printing system for hospitals and physicians to build anatomical models at the point-of-care
Stratasys PolyJet technology and Materialise FDA-cleared software now most versatile 3D printing system for hospitals and physicians to build anatomical models at the point-of-care
MINNEAPOLIS & REHOVOT, Israel--(BUSINESS WIRE)-- Further bringing 3D printed medical models to life, Stratasys (Nasdaq: SSYS) is expanding the suite of printers and materials validated by its collaborator Materialise (Nasdaq: MTLS) as part of FDA-cleared Materialise Mimics inPrint software. The end result is the most versatile 3D printing system for point-of-care across hospitals and physicians - advancing production of patient-specific, life-like anatomical models for diagnostic purposes in conjunction with other tools and expert clinical judgement.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20181126005388/en/
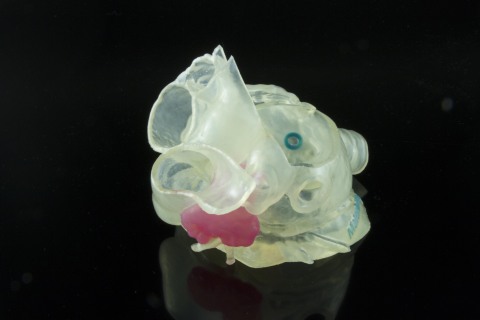
Model of patient's left atrial appendage (LAA) created with Stratasys and Materialise technology is intended to allow surgeons to select the appropriate device and plan the optimal approach to occlude the LAA. Image provided by Materialise.
PolyJet multi-material and multi-color solutions validated now include the J750 and J735 3D Printers and the high-performance desktop Objet30 Prime 3D Printer. Materialise Mimics inPrint is the first and currently only 3D printing software cleared by the FDA to create anatomical models for patient care. The software is designed to allow physicians and hospitals to leverage 3D printing at point-of-care - building a trusted and reliable source for surgical planning and interdisciplinary communication.
“Historically, pre-surgical planning relied on 2D imaging requiring physicians to mentally reconstruct the patient anatomy. But 3D printing evolves this approach by putting precise replicas of patient anatomy directly in physician hands. Our collaboration with Materialise is a huge step towards unlocking the potential of this technology for patient care,” says Eyal Miller, Head of Healthcare Business Unit, Stratasys. “Now the 3D printer that every hospital needs to power their medical modeling comes with additional options for an FDA-cleared software solution.”
In March of 2018, Materialise Mimics inPrint was cleared by the FDA, becoming the only solution with 510(k) clearance as an end-to-end 3D printing solution, as well as a fully comprehensive 3D printing solution for anatomical modeling. According to company reports, of the top 20 U.S. hospitals as ranked by U.S. News & World Report, 16 have implemented a medical 3D printing strategy using Materialise Mimics technology.
“By validating Stratasys’ 3D printing technologies through our certification process, we’re giving doctors and hospitals improved access to high-quality anatomical models for personalized care to patients,” said Bryan Crutchfield, Vice President and General Manager of Materialise North America. “The addition of multi-color and multi-material printers to the list of validated printers is aimed to enable healthcare providers to implement a versatile offering that can support their most complex cases across a wide range of surgical specialties on a single printer. At Materialise, we take a hardware-agnostic approach to software development, offering the flexibility to partner with other leaders in the 3D printing industry like Stratasys – a company committed to addressing requirements of the medical community.”
The Stratasys J735/J750 3D Printers are able to develop highly-complex models using multiple textures, while combining hard and soft materials to mimic human tissue. The unique combination of transparency with multiple color re-creation ensures practitioners can differentiate anatomy, view critical structures within an organ replica, and create realistic representations of any bone, tissue, and organ.
The Stratasys’ Objet30 Prime 3D Printer is a cost-effective, desktop platform providing an entry point to hospitals seeking a point-of-care printing solution - without compromising quality, resolution or accuracy. The versatile offering supports a range of anatomical models and applications, including orthopedic, cardiac, neurosurgery and other use-cases for visualization, surgical planning, training and education.
For more information on how you can integrate Stratasys’ 3D printing solutions and Materialise software to revolutionize medical modeling practices and improve patient outcomes, visit https://www.stratasys.com/medical
Visit Stratasys at RSNA 2018
Stratasys J750 and Objet30 Prime 3D Printers will be at RSNA 2018 at McCormick Place in Chicago, IL from November 25 to November 30. The company is offering exclusive hands-on demos, detailed customer use-cases and presentations throughout the show at Booth # 1968L, South Hall.
Materialise incorporates 27 years of 3D printing experience into a range of software solutions and 3D printing services, which together form the backbone of the 3D printing industry. Materialise’s open and flexible solutions enable players in a wide variety of industries, including healthcare, automotive, aerospace, art and design, and consumer goods, to build innovative 3D printing applications that aim to make the world a better and healthier place. Headquartered in Belgium, with branches worldwide, Materialise combines the largest group of software developers in the industry with one of the largest 3D printing facilities in the world. Further information at: www.materialise.com.
Stratasys is a global leader in additive manufacturing or 3D printing technology, and is the manufacturer of FDM® and PolyJet™ 3D Printers. The company’s technologies are used to create prototypes, manufacturing tools, and production parts for industries, including aerospace, automotive, healthcare, consumer products and education. For 30 years, Stratasys products have helped manufacturers reduce product-development time, cost, and time-to-market, as well as reduce or eliminate tooling costs and improve product quality. The Stratasys 3D printing ecosystem of solutions and expertise includes: 3D printers, materials, software, expert services, and on-demand parts production.
Corporate Headquarters: Minneapolis, Minnesota and Rehovot, Israel. Online at: http://www.stratasys.com/, http://blog.stratasys.com/ and LinkedIn.
Stratasys is a registered trademark and J750, Objet30 Prime, and Stratasys signet are trademarks of Stratasys Ltd. and/or its subsidiaries or affiliates. All other trademarks are the property of their respective owners.
Note Regarding Forward-Looking Statements
The statements in this press release relating to Stratasys’ beliefs regarding the benefits consumers will experience from the Stratasys J750, Objet30 Prime 3D Printers or their validation with Materialise, Stratasys’ expectation on the timing of shipping the Stratasys J750, Objet30 Prime 3D Printers or their validation with Materialise, are forward-looking statements reflecting management's current expectations and beliefs. These forward-looking statements are based on current information that is, by its nature, subject to rapid and even abrupt change. Due to risks and uncertainties associated with Stratasys' business, actual results could differ materially from those projected or implied by these forward-looking statements. These risks and uncertainties include, but are not limited to: the risk that consumers will not perceive the benefits of the Stratasys J750, Objet30 Prime 3D Printers or their validation with Materialise to be the same as Stratasys does; the risk that unforeseen technical difficulties will delay the shipping of the Stratasys J750, Objet30 Prime 3D Printers or their validation with Materialise; and other risk factors set forth under the caption “Risk Factors” in Stratasys' most recent Annual Report on Form 20-F, filed with the Securities and Exchange Commission (SEC) February 28, 2018. Stratasys is under no obligation (and expressly disclaims any obligation) to update or alter its forward-looking statements, whether as a result of new information, future events or otherwise, except as otherwise required by the rules and regulations of the SEC.
Attention Editors, if you publish reader-contact information, please use:
- USA +800-801-6491
- Europe/Middle East/Africa +49-7229-7772-0
- Asia Pacific +852 3944-8888
View source version on businesswire.com: https://www.businesswire.com/news/home/20181126005388/en/
Contacts
Stratasys Media Contacts
Stratasys Corporate & North America
Craig.Librett@stratasys.com
+1 612-364-3208
Europe, Middle East, and Africa
Jonathan Wake / Miguel Afonso, Incus Media
stratasys@incus-media.com
+44 1737 215200
Greater China, Southeast Asia, ANZ, and India
Alice Chiu
media.ap@stratasys.com
+86-21-33196051
Japan and Korea
Aya.Yoshizawa@stratasys.com
+81 3 5542 0042
Mexico, Central America, Caribe and South America
Erica.massini@stratasys.com
+55 11 2626-9229
Brazil
Caio.Ramos@GPcom.com.br
Nando@GPcom.com.br
GP Communications
+55 (11) 3129 5158
Materialise Media Contact:
Virginia Goble
Vice President of Marketing -NA
Materialise
248-921-5500
virginia.goble@materialise.com
Source: Stratasys Ltd.
Smart Multimedia Gallery
Model of patient's left atrial appendage (LAA) created with Stratasys and Materialise technology is intended to allow surgeons to select the appropriate device and plan the optimal approach to occlude the LAA. Image provided by Materialise.
Creating highly-accurate models using multiple textures that mimic human tissue, the Stratasys J750 3D Printer has eight different materials now validated with the Materialise FDA-cleared software. (Photo: Business Wire)








