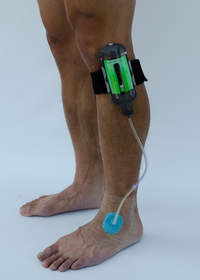
SUNNYVALE, CA--(Marketwire - April 19, 2012) - Spiracur Inc., the developer of an ultraportable and disposable negative pressure wound therapy (NPWT) device, today announced four new clinical abstracts demonstrating the efficacy and clinical value of the SNaP® Wound Care System for the treatment of chronic and acute wounds. Results from one abstract -- a report on the first European experience utilizing the novel NPWT device to treat hard-to-heal chronic wounds -- indicate that use of Spiracur's SNaP System is effective at successfully managing exudate and inducing wound bed granulation tissue in patients with chronic wounds. In addition, patients reported positive results in terms of quality of life and clinical outcomes.
The clinical abstracts, as well as physician presentations on use of Spiracur's ultraportable device, are being highlighted at the Spring Symposium on Advanced Wound Care and Wound Healing Society (SAWC Spring/WHS), April 19-22, 2012 in Atlanta.
"Initial results from our trial suggest that the novel SNaP device may redefine how chronic wounds are treated worldwide," said Professor Keith Harding, director, Institute for Translation, Innovation, Methodologies and Engagement (TIME), Cardiff University School of Medicine in Wales. "These patients are part of an ongoing clinical trial, which includes patients with diabetic foot ulceration, venous leg ulceration and patients with mixed aetiology leg ulcers. The patients all showed reduction in size, level of exudate and an increase in the percentage of granulation tissue in the wound beds. In some patients, wound pain reduced significantly, and all patients reported positive outcomes for quality of life."
In separate research, the Cleveland Clinic evaluated use of the SNaP device for the treatment of acute post surgical incision and drainage wounds, which have not previously been described.
"Based on our clinical experience, we consider the SNaP system to be a valuable addition to our wound care practice," said Michael M. Loor, M.D., FACS, Acute Care Surgeon, Acute Care Surgery, Cleveland Clinic, Cleveland, Ohio. "We found the device to be beneficial to our patients who needed a more portable option for NPWT, as well as a great choice for smaller acute post-surgical wounds that are not typically considered for NPWT, such as contaminated wounds after abscess drainage."
In addition, data was presented on the switch from traditional electrically powered NPWT to Spiracur's ultraportable mechanically powered NPWT, showing significant improvement in patients' satisfaction and comfort. Separate research was also performed to evaluate the use of foam with Spiracur's SNaP System for complete wound closure. Findings from this study illustrate excellent results with foam including decreases in wound size and robust wound bed granulation tissue formation.
"The growing body of research studies utilizing the SNaP System for chronic and acute wound care treatment exemplifies the significance of our innovative technology," said Kenton Fong, M.D., chief medical officer, Spiracur Inc. "The research using our ultraportable SNaP System in ways not traditionally conceived of for powered NPWT devices is particularly promising for improving patient outcomes."
Spiracur will showcase the SNaP Wound Care System with its BLUE Foam Dressing Kit, as well as host in-booth presentations by industry experts at the 25th Annual SAWC/WHS meeting. Considered the premier interdisciplinary wound care program, SAWC/WHS is the largest gathering of wound care clinicians in the United States. It is estimated that more than 2,000 physicians, podiatrists, nurses, therapists and researches will attend the meeting.
About Spiracur Inc.
Spiracur Inc., headquartered in Sunnyvale, Calif., is a privately held medical device company focused on the development of innovative wound healing technologies. Spiracur was founded out of the Stanford Biodesign Innovation Program in 2007. Its first product, the SNaP Wound Care System, is the result of patient and clinician feedback that current negative pressure wound therapies were too cumbersome. The SNaP Wound Care System was cleared by the U.S. Food & Drug Administration (FDA) in August 2009 in a new therapy category defined as "non-powered" NPWT devices, and the company obtained CE Mark for the device in December 2010. For more information, please visit http://www.spiracur.com.
Spiracur, Spiracur logo, SNaP, SNaP & Design and Smart Negative Pressure are registered trademarks of Spiracur Inc.
VAC is a registered trademark of Kinetic Concepts, Inc.
Media Contact:
Amy Cook
925.552.7893
Email Contact
Investor Contact:
Steve Van Dick
408.701.5300
Email Contact