PARIS, Feb. 9, 2016 /PRNewswire/ -- Sanofi (NYSE: SNY; EURONEXT: SAN)
Q4 2015 | Change | Change (CER) | 2015 | Change | Change (CER) | |
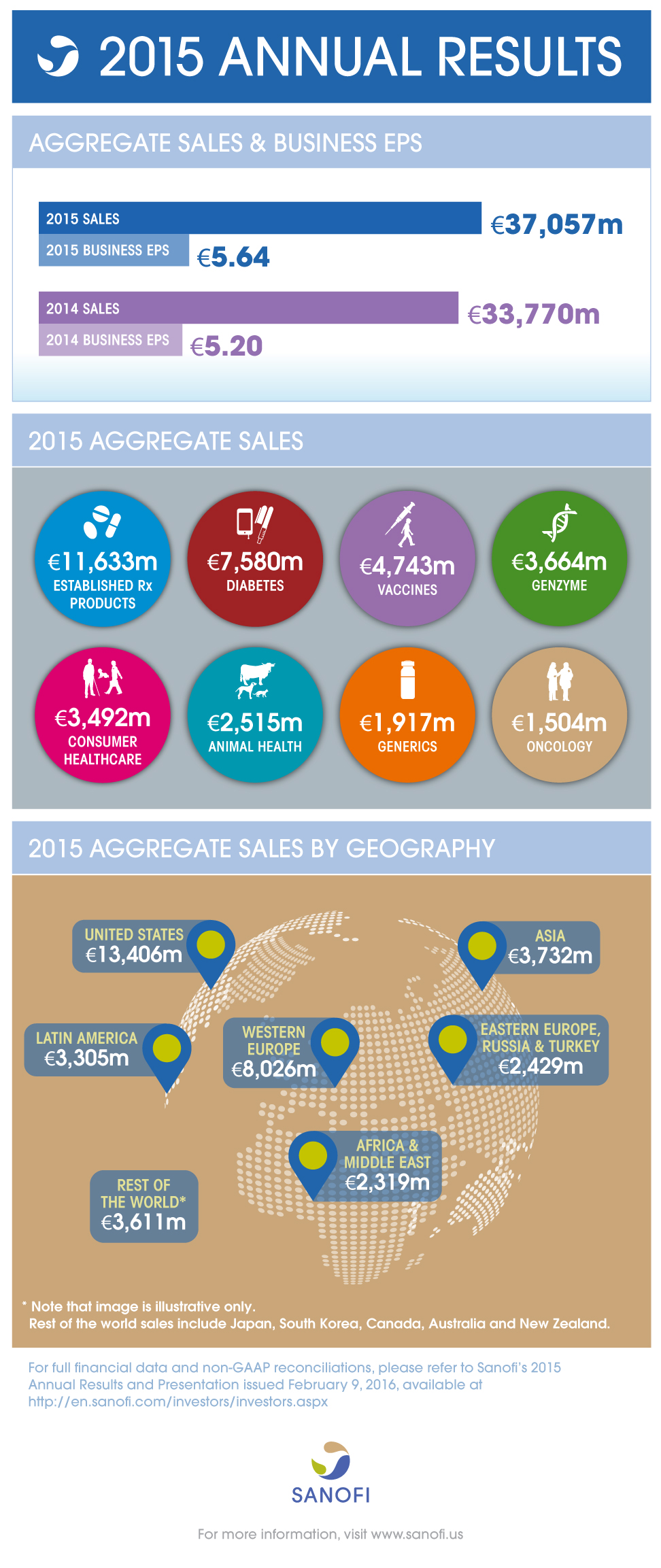
Aggregate Group sales(1) | 9,278m | +2.3% | -1.6% | 37,057m | +9.7% | +2.2% |
Business net income(2) | 1,709m | -6.5% | -13.5% | 7,371m | +7.7% | -0.9% |
Business EPS(3) | 1.31 | -5.8% | -12.9% | 5.64 | +8.5% | 0.0% |
(1) Including Animal Health Business, which is reported on a single line in the consolidated income statements in accordance with IFRS 5 (Non-current assets held for sale and discontinued operations). Additionally, Sanofi comments include Animal Health Business for every income statement line using "Aggregate" wording. (2) In order to facilitate an understanding of operational performance, Sanofi comments on the business net income statement. Business net income is a non-GAAP financial measure. (3) (EPS) Earnings Per Share. | ||||||
Experience the interactive Multimedia News Release here: http://www.multivu.com/players/English/7753751-sanofi-2015-annual-results/

Sanofi Chief Executive Officer, Olivier Brandicourt, commented:
"In 2015, Sanofi made meaningful progress with key launches, multiple business development activities and our efforts to simplify the organization. Entering into exclusive negotiations on a business swap with Boehringer Ingelheim would bring us leadership in CHC. This is a key first step in reshaping our portfolio. In 2016, we continue to allocate resources to our promising late-stage pipeline and the introduction of innovative medicines, which will position us for accelerated future growth."
Executing on 2020 strategic roadmap
- Key milestone in reshaping the portfolio with announcement of exclusive negotiations on business swap
- Significant R&D alliances in oncology and diabetes
- Praluent® launch progressing with significant U.S. market access for 2016
- Toujeo® available globally in over 20 countries, reaching sales of 98 million in Q4 2015
Recent achievements in advancing Sanofi's R&D pipeline of innovative medicines
- Dengvaxia®, the world's first dengue vaccine, approved in Mexico, Brazil, El Salvador and the Philippines
- Biologics License Application for sarilumab accepted for review by the FDA
- FDA submission for priority review of once-daily combination of insulin glargine and lixisenatide
Sales growth in Pharmaceuticals, Vaccines and Animal Health in 2015
- Aggregate Group sales up 2.2% (+9.7% at 2015 exchange rates) to 37,057 million
- Genzyme continues to be a key driver with sales up 29.5% with strong momentum in multiple sclerosis
- Vaccines sales were up 7.3% benefiting from double-digit growth in Emerging Markets
- Diabetes sales decreased 6.8% in line with October guidance, reflecting lower U.S. sales of Lantus®
- Animal Health demonstrated strong performance with sales up 10.8% driven by NexGard®
- Emerging Markets Aggregate sales increased 7.8%, driven by strong growth in China, up 19.5%
Solid financial results in 2015 while making significant investments in new product launches
- Business EPS was 5.64, up 8.5% on a reported basis and stable at CER
- Free Cash Flow up 12.2% to 8,132 million resulting in net debt of 7,254 million
- Board proposes dividend of 2.93, the 22nd consecutive year of dividend growth
2016 financial guidance
- Sanofi expects 2016 Business EPS to be broadly stable at CER, barring unforeseen major adverse events
R&D Update
Regulatory update
Regulatory updates since the publication of the third quarter results on October 29, 2015 include the following:
- In January, Sanofi and Regeneron announced that the U.S. Food and Drug Administration (FDA) accepted for review the Biologics License Application (BLA) for sarilumab. Per the Prescription Drug User Fee Act (PDUFA), the target action date is Oct. 30, 2016.
- In December, Dengvaxia® was granted regulatory approval by the regulatory authorities in Brazil, Mexico and the Philippines.
- In December, the CHMP granted a Positive Opinion recommending the Marketing Authorization of PR5I, Vaxelis, in EU.
- In December, Sanofi submitted a New Drug Application (NDA) to the FDA for its investigational fixed-ratio combination of insulin glargine 100 Units/mL and lixisenatide, which if approved would be administered as a single daily injection for the treatment of adults with type 2 diabetes.
At the beginning of February 2016, the R&D pipeline contained 46 pharmaceutical new molecular entities (excluding Life Cycle Management) and vaccine candidates in clinical development of which 14 are in Phase III or have been submitted to the regulatory authorities for approval.
Collaboration
- In January, Sanofi and Warp Drive Bio, a privately held biotechnology company using the molecules and mechanisms of nature to discover and develop transformative medicines, announced that they have extended and reshaped their existing collaboration utilizing Warp Drive's proprietary SMART (Small Molecule Assisted Receptor Targeting) and Genome Mining platforms to discover novel oncology therapeutics and antibiotics.
- In January, Sanofi and Innate Pharma announced that they have entered into a research collaboration and licensing agreement to apply Innate Pharma's new proprietary technology to the development of innovative bispecific antibody formats engaging natural killer (NK) cells to kill tumor cells through the activating receptor NKp46.
- In November, Sanofi and Lexicon entered into a collaboration and license agreement for the development and commercialization of sotagliflozin, an investigational new oral dual inhibitor of sodium-glucose cotransporters 1 and 2 (SGLT-1 and SGLT-2), which could be a potential treatment option for people with diabetes. The developmental medicine sotagliflozin (LX4211) is currently being studied in two pivotal Phase III trials in type 1 diabetes, which are expected to report top-line results during the second half of 2016. Phase III trials in type 2 diabetes are expected to begin in 2016.
- In November, Sanofi and Hanmi announced a worldwide license agreement to develop a portfolio of experimental, long-acting diabetes treatments. Sanofi has obtained an exclusive worldwide license to develop and commercialize efpeglenatide, a late-stage long-acting glucagon-like peptide-1 receptor agonist (GLP1-RA); a weekly insulin and; a fixed-dosed weekly GLP-1-RA/insulin drug combination.
- Sanofi and BioNTech A.G. announced in November that they entered into a multiyear exclusive collaboration and license agreement. This research collaboration between Sanofi and BioNTech will leverage the scientific expertise of the two organizations to discover and develop up to five cancer immunotherapies, each consisting of a mixture of synthetic messenger RNAs (mRNAs).
- In November, Sanofi and AstraZeneca announced a direct exchange of 210,000 compounds from their respective, proprietary compound libraries. The exchange represents a novel, open innovation model of collaboration between two leading pharmaceutical companies. It will enhance the chemical diversity of the compound collections of both companies and allow each company to screen a broader, more diverse chemical space as the starting point in the search for new, small-molecule medicines.
To access the full press release of the 2015 annual results, please click here.
About Sanofi
Sanofi, a global healthcare leader, discovers, develops and distributes therapeutic solutions focused on patients' needs. Sanofi has core strengths in diabetes solutions, human vaccines, innovative drugs, consumer healthcare, emerging markets, animal health and Genzyme. Sanofi is listed in Paris (EURONEXT: SAN) and in New York (NYSE: SNY).
Sanofi is the holding company of a consolidated group of subsidiaries and operates in the United States as Sanofi US. For more information on Sanofi US, please visit http://www.sanofi.us and http://www.news.sanofi.us/social-media or call 1-800-981-2491.
Forward-Looking Statements
This press release contains forward-looking statements as defined in the Private Securities Litigation Reform Act of 1995, as amended. Forward-looking statements are statements that are not historical facts. These statements include projections and estimates and their underlying assumptions, statements regarding plans, objectives, intentions and expectations with respect to future financial results, events, operations, services, product development and potential, and statements regarding future performance. Forward-looking statements are generally identified by the words "expects", "anticipates", "believes", "intends", "estimates", "plans" and similar expressions. Although Sanofi's management believes that the expectations reflected in such forward-looking statements are reasonable, investors are cautioned that forward-looking information and statements are subject to various risks and uncertainties, many of which are difficult to predict and generally beyond the control of Sanofi, that could cause actual results and developments to differ materially from those expressed in, or implied or projected by, the forward-looking information and statements. These risks and uncertainties include among other things, the uncertainties inherent in research and development, future clinical data and analysis, including post marketing, decisions by regulatory authorities, such as the FDA or the EMA, regarding whether and when to approve any drug, device or biological application that may be filed for any such product candidates as well as their decisions regarding labelling and other matters that could affect the availability or commercial potential of such product candidates, the absence of guarantee that the product candidates if approved will be commercially successful, the future approval and commercial success of therapeutic alternatives, the Group's ability to benefit from external growth opportunities, trends in exchange rates and prevailing interest rates, the impact of cost containment initiatives and subsequent changes thereto, the average number of shares outstanding as well as those discussed or identified in the public filings with the SEC and the AMF made by Sanofi, including those listed under "Risk Factors" and "Cautionary Statement Regarding Forward-Looking Statements" in Sanofi's annual report on Form 20-F for the year ended December 31, 2014. Other than as required by applicable law, Sanofi does not undertake any obligation to update or revise any forward-looking information or statements.
Media Relations:
Mary Kathryn Steel
908-989-0726
USMediaRelations@sanofi.com
Investor Relations:
George Grofik
908-981-5560
IR@sanofi.com


To view the original version on PR Newswire, visit:http://www.prnewswire.com/news-releases/sanofi-delivered-2015-business-eps-up-85-on-a-reported-basis-and-stable-at-constant-exchange-rates-consistent-with-guidance-300217264.html
SOURCE Sanofi





