GLEN ALLEN, VA--(Marketwire - January 30, 2012) -
| Highlighted Links |
http://www.anatabloc.com |
http://www.starscientific.com |
Anatabloc(TM) on Facebook |
Phase 1 of the study was a one-day trial to assess whether Anatabloc™ was as successful as CigRx®, the company's other dietary supplement, in reducing the urge to smoke, and results showed that CigRx® and Anatabloc™ were equally effective. Phase 2 of the study was an open-label extension in which subjects were instructed to take two Anatabloc™ tablets three times per day for two weeks. Study site visits were scheduled at the end of each one-week period so that subjects could complete study assessments and for collection of blood and breath samples. In Phase 2, subjects continued to smoke, but took 6 tablets per day of Anatabloc™. Phase 3, a longer open-label extension, is ongoing.
Phase 2 data analysis examined the CRP and anatabine levels in the subjects' blood, first to establish a baseline, and then to assess levels with Anatabloc™ dosing:
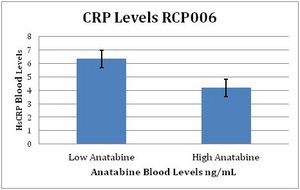
The graph shown on the right illustrates that CRP levels in subjects with dose-appropriate anatabine levels were about 30% lower than CRP levels among those with low anatabine levels. These levels were documented despite subjects' continued smoking, and at a relatively low dose of anatabine by bodyweight in some very heavy subjects (79% were overweight or obese).
Paul L. Perito, Rock Creek's Chairman and CEO, commented, "These findings are encouraging as well as fascinating. This clinical trial clearly demonstrates there is a real and significant effect on CRP levels in blood from nutritional supplementation with Anatabloc™." Curtis Wright MD/MPH, Senior VP for Rock Creek Pharmaceuticals, commented, "CRP is a highly variable measure that is difficult to work with. I am very pleased that we were able to use modern analyses to understand this complex data, thanks to analytical assistance from the Roskamp Institute. I am amazed that these low doses of Anatabloc™ had an effect in this population, which is at such high risk due to obesity and smoking."
Anatabloc™ was introduced in August, 2011 and has been available for purchase via the company's dedicated marketing program that includes a toll-free telephone ordering system, a dedicated product website and one e-commerce web portal. Visit www.anatabloc.com to learn more about Anatabloc™ and inflammation.
Certain statements contained in this release constitute forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. Such statements include, but are not limited to statements identified by words such as "believes," "expects," "anticipates," "estimates," "intends," "plans," "targets," "projects" and similar expressions. The statements in this release are based upon the current beliefs and expectations of the our company's management and are subject to significant risks and uncertainties. Actual results may differ from those set forth in the forward-looking statements. Numerous factors could cause or contribute to such differences, including, but not limited to, the challenges inherent in new product development initiatives, including the continued development and market acceptance of our nutraceutical and low-TSNA tobacco products, our ability to license and protect our intellectual property, our ability to raise additional capital in the future that is necessary to maintain our business, changes in government policy and/or regulation, including with respect to our nutraceutical and low-TSNA tobacco products, as well as other risks discussed from time to time in our filings with the Securities and Exchange Commission, including, without limitation, our annual report on Form 10-K for the fiscal year ended December 31, 2010. We undertake no duty to update any forward-looking statement or any information contained in this press release or in other public disclosures at any time.
About Star Scientific
Star Scientific is a technology-oriented company with a mission to reduce the harm associated with tobacco at every level. It is engaged in the development of dissolvable smokeless tobacco products that deliver fewer carcinogenic toxins, principally through the utilization of the innovative StarCured® tobacco curing technology. Its subsidiary, Rock Creek Pharmaceuticals, Inc., is involved in the development of nutraceuticals as well as products to address neurological and mood disorders. Rock Creek Pharmaceuticals has scientific and research offices in Gloucester, MA, and a regulatory office in Washington, D.C. Star Scientific has a Corporate and Sales Office in Glen Allen, VA, an Executive, Scientific & Regulatory Affairs office in Bethesda, MD, and a manufacturing facility in Chase City, VA.
Contact:
Sara Troy Machir
Vice President, Communications & Investor Relations
Email Contact
(301) 654-8300


 Digg this
Digg this Bookmark with del.icio.us
Bookmark with del.icio.us Add to Newsvine
Add to Newsvine


