INDIANAPOLIS, July 15, 2016 /PRNewswire/ -- Point-of-care and patient self-testing (PST) are growing standards of care for patients requiring regular PT/INR tests for warfarin therapy. These convenient forms of testing help patients adhere to their prescribed testing frequency and healthcare professionals make more timely treatment decisions. Patients who adhere to their testing schedule spend more time in their therapeutic range, which results in lower incidence of stroke1 or bleeding.2 Additionally, 5060% of patients remain in their target range if monitoring of INR occurs monthly, 7785% if monitored weekly and up to 92% if monitored every three days.3 Patients who spend a high proportion of time (> 70%) in therapeutic range achieve better clinical outcomes.4,5
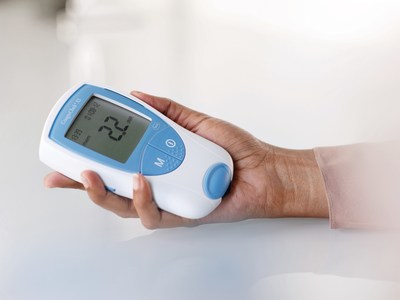
"Patients and caregivers are often pleased to learn that they can test at home rather than have blood drawn in a lab. In appropriately selected patients, CoaguChek PT/INR testing provides precise, accurate results, time savings and convenience" said Dr. Alan Wright, Chief Medical Officer, Roche Diagnostics Corporation.
With PT/INR testing and associated devices in the news, it's important to note the following:
- Roche is committed to continuing to support patients, caregivers and healthcare professionals by supplying CoaguChek PT/ INR meters. Roche is extremely confident in the quality, accuracy, safety and value of CoaguChek PT/INR meters;
- The CoaguChek XS system is 97% accurate compared to lab results6;
- CoaguChek meters have been offered in the U.S. for more than 20 years and there are more than one million CoaguChek XS devices in use around the world;
- Over 250,000 CoaguChek XS PT test strips are used globally per day; and
- Nine of the top 10 U.S. integrated health networks (IHNs) choose CoaguChek technology7.
More about anticoagulant therapy
Millions of people worldwide are taking Vitamin K Antagonists (VKAs) such as Coumadin® and warfarin for a variety of indications or conditions, such as atrial fibrillation (AF), deep vein thrombosis (DVT), pulmonary embolism (PE), and the presence of a mechanical heart valve (MHV). The therapeutic effect of a given dose of warfarin varies among individuals and can change over time due to a number of factors, including diet or concomitant medications. Therefore, safe and effective utilization of chronic warfarin therapy requires close monitoring. This test can be performed at home using a small drop of blood from a patient's fingertip. Compared with usual care or management in an anticoagulation clinic, patient self-testing has been shown to result in more time spent in the therapeutic range,8-10 fewer very high or very low INR values,10 fewer thromboembolic events,1,2 fewer major hemorrhages,2 lower mortality2, improved patient quality of life11 and better treatment satisfaction.12
More about PT/INR
Taking the correct dose is crucial for efficient anticoagulation treatment. A PT/INR is measured to determine if the patient is responding appropriately to the given dose of warfarin. A physician will define a suitable therapeutic range depending on the patient's medical condition such as atrial fibrillation, a blood clot in the leg or lung or a mechanical heart valve. When the INR is higher than the recommended range, it means blood clots more slowly than desired and a lower INR means blood clots more quickly than desired.
About PT/INR monitoring
Historically, management of patients on warfarin therapy included up to weekly visits to the lab with a venipuncture and 2-3 day wait for the lab results. CoaguChek point-of-care and patient self-testing solutions offer lab-like quality and require only a simple finger-stick and 60-second test time.
About PT/INR patient self-testing
In the US, patients may have insurance coverage for self-testing. Often this requires being prescribed or enrolled with a self-testing service provider (an "Independent Diagnostic Testing Facility"). That service will provide face-to-face training for the patient and provides the meter and supplies on an ongoing basis. The service also collects and shares patient PT/INR results with their healthcare team so that adjustments can be made as needed. Roche offers this service through CoaguChek Patient Services. Patients who use that service are guaranteed to receive a CoaguChek meter. Additionally, CoaguChek Patient Services recently introduced secure, wireless connectivity options that give patients even greater independence while maintaining connections with their healthcare providers.
About Roche
Roche is a global pioneer in pharmaceuticals and diagnostics focused on advancing science to improve people's lives.
Roche is the world's largest biotech company, with truly differentiated medicines in oncology, immunology, infectious diseases, ophthalmology and diseases of the central nervous system. Roche is also the world leader in in vitro diagnostics and tissue-based cancer diagnostics, and a frontrunner in diabetes management. The combined strengths of pharmaceuticals and diagnostics under one roof have made Roche the leader in personalized healthcare a strategy that aims to fit the right treatment to each patient in the best way possible.
Founded in 1896, Roche continues to search for better ways to prevent, diagnose and treat diseases and make a sustainable contribution to society. Twenty-nine medicines developed by Roche are included in the World Health Organization Model Lists of Essential Medicines, among them life-saving antibiotics, anti-malarials and cancer medicines. Roche has been recognized as the Group Leader in sustainability within the Pharmaceuticals, Biotechnology & Life Sciences Industry seven years in a row by the Dow Jones Sustainability Indices.
The Roche Group, headquartered in Basel, Switzerland, is active in over 100 countries and in 2015 employed more than 91,700 people worldwide. In 2015, Roche invested CHF 9.3 billion in R&D and posted sales of CHF 48.1 billion. Genentech, in the United States, is a wholly owned member of the Roche Group. Roche is the majority shareholder in Chugai Pharmaceutical, Japan. For more information, please visit www.roche.com.
For more information, please contact:
| |
Roche Jenna Eup, Communications US Phone: 317-521-4477 Email: jenna.eup@roche.com |
All trademarks used or mentioned in this release are protected by law.
References
1. Heneghan C, Ward A, Perera R, et al. Self-monitoring of oral anticoagulation: systematic review and meta-analysis of individual patient data. Lancet. 2012;379:322-334.
2. Heneghan, C., Alonso-Coello, P., Garcia-Alamino, J.M., Perera, R., Meats, E., Glasziou, P. (2006). Self-monitoring of oral anticoagulation: a systematic review and meta-analysis. Lancet 367, 404411.
3. Khan TI, Kamali F, Kesteven P, Avery P, Wynne H. The value of education and self-monitoring in the management of warfarin therapy in older patients with unstable control of anticoagulation. Br J Haematol. 2004;126(4):557-654.
4. Gallagher, A.M., Setakis, E., Plumb, J.M., Clemens, A., van Staa, T.-P. (2011). Risks of stroke and mortality associated with suboptimal anticoagulation in atrial fibrillation patients. Thromb Haemost 106, 968977.
5. Wan, Y., Heneghan, C., Perera, R., Roberts, N., Hollowell, J., Glasziou, P. et al. (2008). Anticoagulation control and prediction of adverse events in patients withatrial fibrillation: a systematic review. Circ Cardiovasc Qual Outcomes 1, 8491.
6. 97% correlation with lab results using Dade Innovin reagent. See package insert for more information. CoaguChek XS PT Test [package insert 05967694001(05)]. Indianapolis, Ind.: Roche Diagnostics; 2016.
7. GHX Market Intelligence. Data on file. Roche Diagnostics.
8. Bereznicki, L.R.E., Jackson, S.L., Peterson, G.M. (2013). Supervised patient self-testing of warfarin therapy using an online system. J Med Internet Res 15, e138.
9. Christensen, H., Lauterlein, J.-J., Sørensen, P.D., Petersen, E.R.B., Madsen, J.S., Brandslund, I. (2011). Home management of oral anticoagulation via telemedicine versus conventional hospital-based treatment. Telemed J E-Health Off J Am Telemed Assoc 17, 169176.
10. Bussey, H.I., Bussey M., Bussey-Smith K.L., Frei, C.R. (2013). Evaluation of warfarin management with international normalized ratio self-testing and online remote monitoring and management plus low-dose vitamin k with genomic considerations: a pilot study. Pharmacotherapy 33, 11361146.
11. Matchar, D.B., Jacobson, A., Dolor, R., Edson, R., Uyeda, L., Phibbs, C.S., et al.; THINRS Executive Committee and Site Investigators. (2010). Effect of home testing of international normalized ratio on clinical events. N Engl J Med 363, 16081620.
12. Gardiner, C., Williams, K., Mackie, I.J., Machin, S.J., Cohen, H. (2005). Patient self-testing is a reliable and acceptable alternative to laboratory INR monitoring. Br J Haematol 128, 242247.

Photo - http://photos.prnewswire.com/prnh/20160715/390069
Logo - http://photos.prnewswire.com/prnh/20160715/390070LOGO
To view the original version on PR Newswire, visit:http://www.prnewswire.com/news-releases/coaguchek-pt-inr-devices-for-use-at-the-point-of-care-and-for-patient-self-testing-remain-a-safe-and-effective-option-for-patients-on-anticoagulation-therapy-300299449.html
SOURCE Roche Diagnostics





