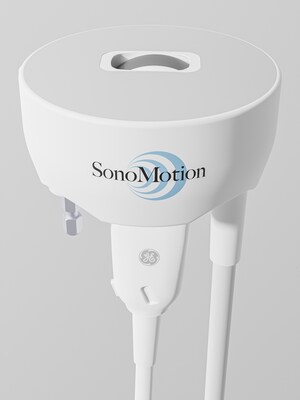
- Break Wave represents the future of kidney stone treatment with the potential to become a first-line treatment option
- The Break Wave device non-invasively fragments stones in the kidney or ureter on fully awake patients, without anesthesia, and at any site of care
- Kidney stones affect one in 10 people in the U.S., costing $10 billion annually and resulting in over 750,000 lithotripsy procedures per year1
SAN MATEO, Calif., Jan. 21, 2026 /PRNewswire/ -- SonoMotion, a venture-backed medical device company developing non-invasive solutions for kidney stones, announced today that it received U.S. Food and Drug Administration (FDA) 510(k) clearance for its Break Wave lithotripsy device. Break Wave uses low pressure-focused ultrasound to fragment kidney stones by creating standing stress waves within the stone under real-time ultrasound image guidance. The procedure is completely non-invasive and requires no anesthesia, allowing patients to drive themselves to and from the procedure and eat and drink prior to the procedure.
"Break Wave provides a new option for the safe and effective treatment of kidney stones that can be performed in nearly any healthcare setting and does not require a ureteral stent," said Helena Chang, MD, Kaiser Permanente. "Patients with symptomatic obstructing ureteral stones can move immediately to treatment, saving weeks of pain and discomfort trying to pass a stone. Additionally, patients with asymptomatic kidney stones have an option to treat stones before they cause a painful event."
SonoMotion is developing and commercializing a kidney stone treatment platform with several devices, including Break Wave and Stone Clear™. Together, the two devices provide an anesthesia-free and non-invasive treatment pathway: Break Wave fragments the stone, while Stone Clear helps clear the kidney of residual fragments post-lithotripsy. Stone Clear was granted FDA 510(k) de novo clearance in October 2024.
"Receiving FDA clearance is a pivotal milestone for our company and, more importantly, for patients seeking better options for kidney stone treatment," said Oren Levy, PhD, Co-Founder and Chief Executive Officer of SonoMotion. "This 510(k) clearance represents a significant step toward commercialization, and we look forward to scaling manufacturing and making our non-invasive, anesthesia-free solutions available to patients and providers across the urology community. We are deeply grateful to the patients who participated in our studies, as well as the support from clinicians, investors, NASA, and the NIH."
The SonoMotion platform, including Break Wave and Stone Clear, will be featured at booth #1508 during the upcoming 2026 American Urological Association (AUA) meeting, May 15-18 in Washington, DC.
ABOUT SONOMOTION
SonoMotion, a VC-backed startup based in San Mateo, CA, is a medical technology company developing next-generation non-invasive solutions for the treatment of kidney stones. To learn more, please visit www.sonomotion.com.
References:
- Litwin MS, CS S. NIH Publication No. 12-7865.
![]() View original content to download multimedia:https://www.prnewswire.com/news-releases/sonomotion-announces-fda-clearance-for-its-break-wave-lithotripsy-device-for-treatment-of-kidney-stones-302666229.html
View original content to download multimedia:https://www.prnewswire.com/news-releases/sonomotion-announces-fda-clearance-for-its-break-wave-lithotripsy-device-for-treatment-of-kidney-stones-302666229.html
SOURCE SonoMotion, Inc.