Reductions in airway hyperresponsiveness and suppressed eosinophilic response at both 6 weeks and 12 weeks observed after a single 180mg dose of Briquilimab in the ETESIAN Study
Preliminary data from ETESIAN study supports further development of briquilimab in asthma
Jasper also announces completion of internal BEACON study investigation noting no deviations or issues with drug product utilized
Jasper to host conference call and webinar today at 8:00 a.m. ET
REDWOOD CITY, Calif., Dec. 02, 2025 (GLOBE NEWSWIRE) -- Jasper Therapeutics, Inc. (Nasdaq: JSPR) (Jasper), a clinical stage biotechnology company focused on development of briquilimab, a novel antibody therapy targeting KIT (CD117) to address mast cell driven diseases such as chronic spontaneous urticaria (CSU), chronic inducible urticaria (CIndU) and asthma, reported positive preliminary clinical data from Jasper’s ETESIAN Phase 1b study of subcutaneous briquilimab in adult participants with allergic asthma. A single subcutaneous 180mg dose of briquilimab demonstrated substantial reductions in sputum eosinophils at both six and twelve weeks, as well as improvements over baseline in FEV1 in both Early Asthmatic Response (EAR) and Late Asthmatic Response (LAR). Significant reductions in serum tryptase were observed, consistent with reductions observed in other briquilimab studies at the 180mg dose level. Briquilimab was well tolerated in the study, demonstrating a favorable safety profile.
Jasper also announced the completion of its internal investigation into the anomalous lack of clinical response observed in the July 2025 BEACON data for cohort 8 (240mg Q8W) and cohort 9 (240mg /180mg Q8W), where no US patients (n=10) achieved Complete Response or Well Controlled UAS7 by week 12. Based on the work completed, the additional data from subsequent dosing of the US patients and input from a panel of CSU KOLs, Jasper has concluded that the unexpected efficacy results observed in the US patients was not the result of any issues with the drug product. Rather, it was likely a result of patient selection issues, as it appears that 9 of the 10 patients in question did not have CSU as their symptoms were not mast cell-driven.
“I am pleased to see the initial results of the ETESIAN study, the first clinical study to evaluate an agent specifically targeting mast cells in asthma patients” said Dr. Elliot Israel, Director of Clinical Research in the Pulmonary and Critical Care Division at the Brigham and Women’s Hospital in Boston. “The initial results demonstrate the potential to reduce both airway hyperresponsiveness and the accumulation of eosinophils in the airways, both of which are key factors in managing chronic asthma and reducing exacerbations. Given that a substantial portion of asthma patients remain underserved by currently approved therapies, depleting mast cells through KIT inhibition may represent an intriguing new treatment option for patients with chronic asthma.”
“We are very pleased to present the positive preliminary data from the ETESIAN study, which demonstrates proof of concept for mast cell depletion using briquilimab as a potential therapeutic option for patients with asthma,” said Dr. Daniel Adelman, Acting Chief Medical Officer of Jasper. “Mast cells are believed to be a key driver of the inflammatory cascade underlying chronic asthma, and both the reductions in airway hyperresponsiveness and the significant reduction in sputum eosinophils demonstrated at 6 weeks after a single 180mg dose of briquilimab strongly support the potential of mast cell depletion to drive a therapeutic benefit for asthma patients. These data, combined with the favorable safety profile observed in the ETESIAN study and in other briquilimab clinical studies, provide a strong rationale for further development of briquilimab in asthma. On behalf of the entire Jasper team, I’d like to thank the investigators and the patients who participated in the study, along with their families and caregivers.”
ETESIAN Study Design and Preliminary Data Summary:
The Phase 1b/2a ETESIAN study was a single dose double-blind, placebo-controlled challenge study that enrolled approximately 17 patients across 6 sites in Canada. The primary objective of the study was to demonstrate proof-of-concept for briquilimab in asthma utilizing a potentially therapeutic dose to inform future trials in the broader asthma population. The study was conducted utilizing a single 180mg dose of subcutaneous briquilimab and key assessments included both EAR measured at 6 weeks, and LAR measured at 12 weeks, changes in airway hyperresponsiveness, mast cell depletion and recovery, and safety.
The preliminary data includes the results from 14 participants, 8 receiving a single dose of 180mg briquilimab and 6 receiving placebo, who completed at least 6 weeks of allergen challenge testing following initial dosing with investigational product. Compared to baseline, briquilimab reduced the allergen induced LAR (measured by the mean maximum percentage fall in FEV1 (%Max FEV1) and fall in area under the FEV1 time response curve (AUC)) at both 6 and 12 weeks. Patients who received briquilimab showed an improvement in the LAR %Max FEV1 of 10.4% at 6 weeks and 8.7% at 12 weeks compared to baseline and an improvement in the LAR AUC of 25.4% at 6 weeks and 23.3% at 12 weeks.
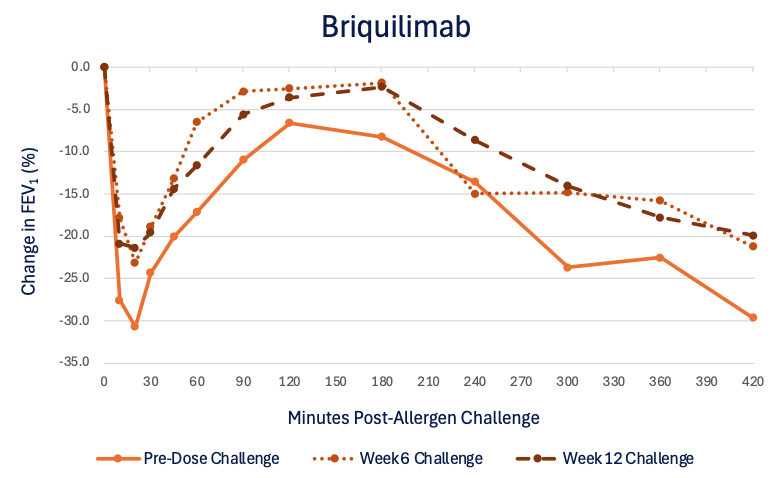
180mg Single-Dose Asthmatic Response Assessments at Week 6 and Week 12
| 6-Week Change (Baseline to Day 42) | 12-Week Change (Baseline to Day 84) | ||||
| % Max FEV1 | AUC | % Max FEV1 | AUC | ||
| Early Asthmatic Response, 0-2 Hours (EAR) | |||||
| Briquilimab(n=8) | 12.74 | 18.27 | 8.13 | 10.56 | |
| Placebo(n=6) | 9.88 | 5.93 | 6.16 | 3.89 | |
| Late Asthmatic Response, 2-7 Hours (LAR) | |||||
| Briquilimab(n=7) | 10.37 | 25.35 | 8.69 | 23.34 | |
| Placebo(n=5) | 3.13 | 9.35 | 0.20 | 1.92 | |
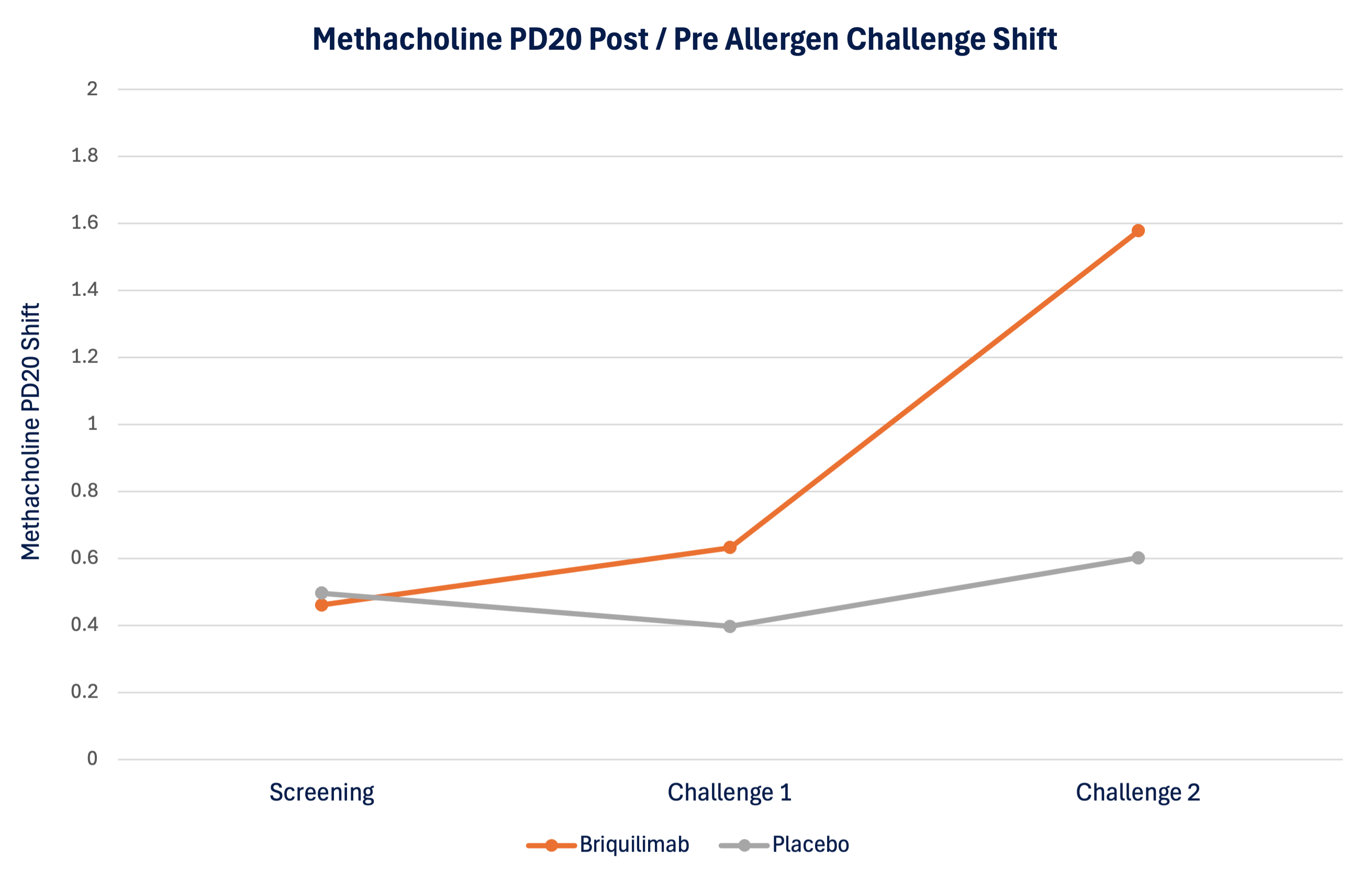
Patient airway hyperresponsiveness was also assessed pre- and post-allergen challenge by methacholine PD20. At baseline, prior to administration of briquilimab, patients randomized to both the placebo group and the briquilimab group had similar drops in the ratio of post- to pre-allergen challenge methacholine PD20 (dose of methacholine required to drive a 20% decrease in FEV1) following allergen challenge of 0.50 and 0.46, respectively. At the week 6 challenge the shift in the methacholine PD20 response was 0.40 for placebo and 0.63 for briquilimab and at the week 12 challenge the shift was 0.60 for placebo and 1.58 for briquilimab indicating an increased resistance to methacholine following allergen in patients dosed with briquilimab.
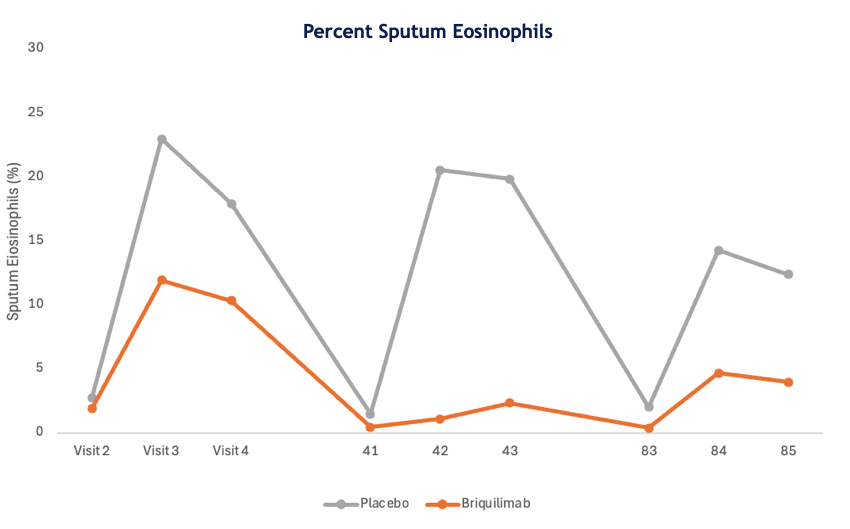
Treatment with briquilimab also decreased levels of sputum eosinophils before and after allergen challenge. Patients in the briquilimab group had reduced mean sputum eosinophil levels at pre allergen challenge timepoints of 1.88% at baseline to 0.44% at day 41 and 0.38% at day 83. Briquilimab also reduced mean sputum eosinophil levels 24 hours following allergen challenge. At baseline, prior to briquilimab dosing, patients randomized to the briquilimab group had a 24-hour post allergen challenge eosinophil level of 10.3%. Following dosing of briquilimab the 24-hour post allergen challenge eosinophil level was 2.32% at 6 weeks and 3.98% at 12 weeks.
Briquilimab was well-tolerated in the study, with no dose limiting toxicities observed. Safety observations potentially related to KIT blockade were infrequent and generally limited to low grade events, none of which resulted in discontinuations or dose delays and the majority of which resolved during repeat dosing.
BEACON Cohort 8 & Cohort 9 Internal Investigation Concluded
Jasper announced the completion of its internal investigation into the anomalous lack of clinical response observed in the July 2025 BEACON data for cohort 8 (240mg Q8W) and cohort 9 (240mg/180mg Q8W), where no US patients (n=10) achieved Complete Response or Well Controlled UAS7 by week 12. In response, Jasper’s internal investigation included:
- switching all US patients to a new lot of drug product for the remainder of their doses on study to determine if drug product played a role,
- a comprehensive review of all manufacturing records, drug handling, site training/ logs and data handling,
- recovery and testing by Jasper and independent labs of drug product samples from across the supply chain,
- a review of all US sites and all US patients, including protocol adherence patient medical histories, patient screening and all pharmacokinetics, pharmacodynamics and efficacy data, and
- assembling a KOL panel to review the internal investigation findings, including full patient dossiers, which provided its input and conclusions from the findings.
The investigation has been completed, and based on the work completed, the additional data from subsequent dosing of the US patients and input from the KOL panel, Jasper has concluded that the unexpected lack of efficacy observed in the US patients was not the result of any issues with drug product, but rather appears to be the result of patient selection issues, specifically the fact that it appears that 9 of the 10 patients did not have CSU as their disease did not appear to be mast-cell driven. This is specifically evidenced by the fact that 8 of the 9 patients continued to demonstrate consistent pharmacodynamic responses despite no improvement in UAS7 scores.
“I commend the Jasper team for the professional manner in which they managed the anomalous results received in July, by promptly notifying clinical sites and conducting a thorough investigation into the root cause,” said Martin Metz, M.D., Professor of Dermatology and Allergy Charité – Universitätsmedizin Berlin and member of the KOL panel. “While it appears that 9 of the 10 patients enrolled in the US sites likely did not have mast cell-driven CSU, their data still provide valuable insights into the pharmacodynamics and the safety profile of briquilimab. I’m very encouraged with the overall profile of briquilimab to date, and I look forward to seeing additional data in early 2026.”
“We are very pleased to be able to close out our internal investigation of the anomalous results seen in the BEACON data released in July and very grateful to Dr. Metz and the other KOLs who supported the rapid completion of this effort,” said Ronald Martell, President and Chief Executive Officer of Jasper. “Going forward, we are confident that the learnings from this investigation and the recommendations from our KOLs will help us minimize the enrollment of patients that may not have mast cell-driven disease. Most importantly, we are very pleased that the internal investigation demonstrated that there were no issues with the drug product utilized in the study, and we are looking forward to the last wave of BEACON data expected in Q1 2026 that will enable us to select final doses for the Phase 2b CSU study, planned to commence mid-2026.”
Conference Call / Webinar
Jasper will host a conference call and webinar today at 8:00 a.m. ET, including remarks from Dr. Martin Metz, M.D., Professor of Dermatology and Allergy Charité – Universitätsmedizin Berlin and the principal European investigator on the BEACON study. A live question and answer session with management will follow the formal presentation. A link to the webinar, including presentation slides, can be found here.
The presentation slides and a link to the live and archived webcast will also be available on the Events & News – Events page of Jasper's Investor Relations website.
About Jasper
Jasper is a clinical-stage biotechnology company focused on developing briquilimab as a therapeutic for chronic mast cell diseases. Briquilimab is a targeted aglycosylated monoclonal antibody that blocks stem cell factor from binding to the cell-surface receptor KIT, thereby inhibiting signaling through the receptor. This inhibition disrupts the critical survival signal, leading to the depletion of the mast cells via apoptosis which removes the underlying source of the inflammatory response in mast cell driven diseases such as chronic urticaria and asthma. Jasper is currently conducting clinical studies of briquilimab as a treatment in patients with CSU, CIndU, and asthma. Briquilimab has a demonstrated efficacy and safety profile in patients and healthy volunteers, with positive clinical outcomes in both CSU, CIndU and asthma. For more information, please visit us at www.jaspertx.com.
Forward-Looking Statements
Certain statements included in this press release that are not historical facts are forward-looking statements for purposes of the safe harbor provisions under the United States Private Securities Litigation Reform Act of 1995. Forward-looking statements are sometimes accompanied by words such as “believe,” “may,” “will,” “estimate,” “continue,” “anticipate,” “intend,” “expect,” “should,” “would,” “plan,” “predict,” “potential,” “seem,” “seek,” “future,” “outlook” and similar expressions that predict or indicate future events or trends or that are not statements of historical matters. These forward-looking statements include, but are not limited to, statements regarding briquilimab’s potential, including with respect to its potential in mast cell driven diseases such as CSU, CIndU and asthma, its potential to reduce both airway hyperresponsiveness and the accumulation of eosinophils in the airways of asthma patients and its potential as a new treatment option for patients with asthma; briquilimab’s safety profile; Jasper’s internal investigation into the anomalous lack of clinical response observed in the July 2025 BEACON data for cohort 8 (240mg Q8W) and cohort 9 (240mg /180mg Q8W); the potential of mast cell depletion to drive a therapeutic benefit for asthma patients; the further development of briquilimab in asthma; Jasper’s expectations regarding a registrational program in CSU, including the expected timing of the Phase 2b study and dose selection; Jasper’s expected timing for presenting data from additional BEACON cohorts; and the topics expected to be discussed during the webinar. These statements are based on various assumptions, whether or not identified in this press release, and on the current expectations of Jasper and are not predictions of actual performance. These forward-looking statements are provided for illustrative purposes only and are not intended to serve as, and must not be relied on by an investor as, a guarantee, an assurance, a prediction or a definitive statement of fact or probability. Many actual events and circumstances are beyond the control of Jasper. These forward-looking statements are subject to a number of risks and uncertainties, including general economic, political and business conditions; the risk that the potential product candidates that Jasper develops may not progress through clinical development or receive required regulatory approvals within expected timelines or at all; the risk that clinical trials may not confirm any safety, potency or other product characteristics described or assumed in this press release; the risk that prior test, study and trial results, including preliminary results for the ETESIAN study reported in this press release, may not be replicated in continuing or future studies and trials; the risk that Jasper may be unable to raise capital to continue its operations and continue the BEACON study; the risk that Jasper will be unable to successfully market or gain market acceptance of its product candidates; the risk that prior study results may not be replicated; the risk that Jasper’s product candidates may not be beneficial to patients or successfully commercialized; patients’ willingness to try new therapies and the willingness of physicians to prescribe these therapies; the effects of competition on Jasper’s business; the risk that third parties on which Jasper depends for laboratory, clinical development, manufacturing and other critical services will fail to perform satisfactorily; the risk that Jasper’s business, operations, clinical development plans and timelines, and supply chain could be adversely affected by the effects of health epidemics; the risk that Jasper will be unable to obtain and maintain sufficient intellectual property protection for its investigational products or will infringe the intellectual property protection of others; and other risks and uncertainties indicated from time to time in Jasper’s filings with the SEC, including its Annual Report on Form 10-K for the year ended December 31, 2024 and subsequent Quarterly Reports on Form 10-Q. If any of these risks materialize or Jasper’s assumptions prove incorrect, actual results could differ materially from the results implied by these forward-looking statements. While Jasper may elect to update these forward-looking statements at some point in the future, Jasper specifically disclaims any obligation to do so. These forward-looking statements should not be relied upon as representing Jasper’s assessments of any date subsequent to the date of this press release. Accordingly, undue reliance should not be placed upon the forward-looking statements.
Contacts:
Alex Gray (investors)
Jasper Therapeutics
650-549-1454
agray@jaspertherapeutics.com
Joyce Allaire (investors)
LifeSci Advisors
617-435-6602
jallaire@lifesciadvisors.com
Media:
media@jaspertx.com
Figures accompanying this announcement are available at:
https://www.globenewswire.com/NewsRoom/AttachmentNg/117de8a4-bab1-4396-8eba-9e527a295599
https://www.globenewswire.com/NewsRoom/AttachmentNg/2691f577-e58f-4a0b-8499-9fd494e15ad1
https://www.globenewswire.com/NewsRoom/AttachmentNg/e4987514-9e54-4e0e-b73b-8f940b8aa3ef





