SHANGHAI, Sept. 5, 2024 /PRNewswire/ -- UltraDx Bio is proud to announce that its flagship product, the UD-X™ Fully Automated Single-Molecule Array Fluorescence Immunoassay Analyzer, has received its first clinical registration approval in China. This groundbreaking achievement is now officially listed on the National Medical Products Administration (NMPA) website (Registration Certificate No.: JXZZ 20242220334) and marks a significant milestone in the field of medical diagnostics. The UD-X™ Single-Molecule Analyzer is designed for the ultra-sensitive detection and quantification of trace protein biomarkers in body fluids, reaching sensitivity levels at the fg/ml scale. This technology will be utilized alongside soon-to-be-approved companion assay kits, addressing the precision detection needs of clinical diagnostics and community health screenings.
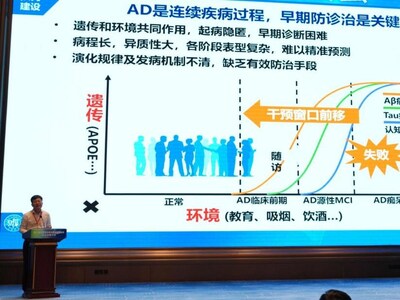
The successful approval and clinical deployment of the UD-X™ Single-Molecule Analyzer are poised to revolutionize early screening, early diagnosis, companion diagnosis and treatment effectiveness evaluation for diseases such as Alzheimer's Disease (AD). This advancement is not only of immense clinical value but also holds significant social and economic importance.
Driven by a passion for innovation and a deep commitment to patient care, UltraDx Bio is dedicated to enhancing its R&D and production capabilities. The company is committed to fostering collaborations with various stakeholders to promote continuous advancements in disease screening, precision diagnostics, and the development of versatile application platforms. Together, we aim to drive the industry's growth and improve global health outcomes.
Product Launch Ceremony
On August 25, 2024, UltraDx Bio hosted the launch ceremony for the UD-X™ Single-Molecule Analyzer. The event was a significant success, with Jay Zengjun Dong, CEO of UltraDx Bio, delivering an inspiring keynote address. The event garnered widespread attention from both industry insiders and external observers, attracting renowned experts and thought leaders who acknowledged the exceptional performance of the single-molecule array technology in both academic and clinical research. They also expressed high expectations for its future applications in early detection and diagnosis of diseases like Alzheimer's.
Distinguished guests included world-renowned key thought leaders, scientists and clinical experts : Academician George Fu Gao, Member of National Academy of Sciences of USA, Member of Royal Academy, and Member of Chinese Academy of Sciences, and president of Chinese Society of Biotechnology and former Director of China CDC in Beijing; Academician Guoliang Xu , the Member of Chinese Academy of Sciences, a research Professor of Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences; Professor Jintai Yu, of National Clinical Center for Neurological diseases in Shanghai, Deputy Director of Neurology of Huashan Hospital Affiliated to Fudan University, Deputy Director of Neurology Research Institute of Fudan University, and Vice Chairman of Neurology Committee, Chinese Medical Association; Professor Yanning Cai, of National Clinical Center for Neurological diseases in Beijing, Director of the Clinical Sample Center of Xuanwu Hospital of Capital Medical University in Beijing and Deputy Director of the Key Project Department of the National Center for Neurological Diseases, and other clinical experts and scholars toured our company clinical and product facility, inspected the company's production process, quality control system, and the progress of the product research and development, and explored the cutting-edge of the precision medicine testing technology. The experts pointed out that with the aggravation of aging population, the incidence of Alzheimer's disease is increasing year by year, which brings a heavy burden to patients, their families and the society. Strengthening the basic and clinical research and clinical application transformation in this field is one of the important tasks and social responsibilities shared by both the clinical side and the industry side.
Subsequently, all invited experts had focused discussion on the topic of "Opportunities and Prospects of Early Detection and early Diagnosis of Diseases", with a focus on AD and cancer, reached a consensus that the low rate of early diagnosis and consultation of diseases represented by Alzheimer's Disease is mainly due to (1) insufficient knowledge of patients about the disease; (2) lack of means of early diagnosis and effective treatment; and (3) the early diagnosis rate is closely related to the development progress of society.
【Expert Opinions】
Academician George Fu Gao, the Member of the Chinese Academy of Sciences, the Foreign Member of the U.S. National Academy of Sciences , the foreign member of the Royal Academy, emphasized that precision diagnosis is the cornerstone of precision medicine, praising UltraDx Bio's efforts in developing advanced diagnostic tools for Alzheimer's disease.
Academician Guoliang Xu, the Member of the Chinese Academy of Sciences, a researcher at the Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences, underscored the necessity of in-depth basic research for technological advancement and the translation of scientific findings into clinical and industrial applications. He further emphasized, "The results of scientific research should also rely on clinical and corporate industry translation to ultimately benefit human beings." "we shall also pay more attention to individual variation."
Prof. Jintai Yu, of National Clinical Center for Neurological diseases in Shanghai, Deputy Director of Department of Neurology, Huashan Hospital Affiliated to Fudan University, Executive Deputy Director of Institute of Neurology, Fudan University, and Vice Chairman of the Youth Committee of Neurology Branch of Chinese Medical Association, highlighted the challenges of early Alzheimer's diagnosis, stressing the importance of early detection and intervention to mitigate disease progression. He clearly pointed out the main challenges of early diagnosis of AD and emphasized that once a patient enters the dementia stage, the existing treatments have limited effect, so early detection and intervention are crucial to slow down the progression of the disease. He pointed to the potential of plasma biomarker precision testing as a solution for large-scale diagnostic needs.
Meanwhile, Professor Yanning Cai, of National Clinical Center for Neurological Diseases in Beijing, Director of the Clinical Sample Center of Xuanwu Hospital of Capital Medical University in Beijing, and Deputy Director of the Key Project Department of the National Center for Neurological Diseases Medicine, Professor Cai Yanning focused on the importance of standardized clinical sample management, advocating for companies to establish comprehensive, standardized processes to ensure seamless clinical application of precision medicine.
Prof. Shukun Yao, PhD supervisor of Peking Union Medical College, Peking University, Beijing University of Traditional Chinese Medicine, former vice president of China-Japan Friendship Hospital, and Chief of Precision Medicine Specialized Committee, said after the academic discussion with Prof. Yu on the commonality, characteristics and differential diagnosis of AD and vascular dementia, "As the clinical side, we hope to join hands with scientific research and enterprises, and make concerted efforts from all sides, so as to contribute to the early diagnosis and early treatment of AD. "
【Important Voices】
Mr. Keith Crandell, Co-founder & Managing Director of ARCH, said, "We are very excited and confident about using Simoa for early diagnosis of AD and other diseases in China. We are fully committed to investing funds and resources to translate this breakthrough technology into products and solutions for research and diagnostics to help patients and their families around the world."
Managing Director of GF Hong Kong Ms. Xiaoying Mai said, "We are excited about this technology and its potential, and are impressed with Jay Dong's passion, energy and leadership. GFHK fully supports this company that can provide a solution to the huge blue ocean market of early AD diagnosis and hopes that patients will soon benefit from this technology."
"We believe that early detection is an important starting point for the treatment of disease. Many AD biomarkers in the blood are not detectable or cannot be detected earlier without the use of ultra-high sensitivity Simoa technology. Our collaboration with UltraDx Bio for IVD product development and commercialization in China will accelerate and improve early diagnosis of Alzheimer's disease patients." Dr. Masoud Toloue, CEO of Quanterix added, "We're excited about the IVD achievement, and a look forward to supporting UltraDX in broadening early Alzheimer's disease testing in China."
"This is only a small step towards achieving a great mission: enabling early diagnosis and early intervention, and making our world a heathier planet", added passionately by Jay Dong, CEO of UltraDx.
About Alzheimer's Disease
Alzheimer's disease is the most common age-related neurodegenerative disorder, severely impacting patients and their families. China has the highest number of Alzheimer's patients globally, with over ten million affected individuals. By 2030, the economic burden associated with Alzheimer's in China is expected to reach nearly one trillion yuan. The Simoa®-based clinical tests developed by UltraDx Bio provide a new, efficient solution to this urgent need, enabling more accurate and early diagnosis for effective intervention and treatment.
About UltraDx Bio
Founded by scientist-entrepreneur Jay Dong, UltraDx Bio is recognized by leading biopharmaceutical venture capital funds, including ARCH and GF HK. The company's products leverage state-of-the-art Simoa® single-molecule array technology, adapted and approved for clinical use in China. With comprehensive capabilities in R&D, translational research, production, clinical registration, and quality management, UltraDx Bio focuses on developing diagnostic tools for neurological, infectious, immune, cardiovascular, and tumor diseases. The company operates R&D and production centers in Shanghai and Shijiazhuang, offering a robust product portfolio that includes AD early diagnostic instruments, reagents, and complete service solutions. By integrating leading global technology platforms with localized strategies and execution, UltraDx Bio is committed to advancing early disease diagnosis and improving human health. For more information, please contact us at info@ultradxbio.com.
![]() View original content to download multimedia:https://www.prnewswire.com/news-releases/a-small-step-towards-a-big-mission-worlds-first-ultradx-received-first-clinical-approval-of-single-molecule-analyzer-in-china-302239416.html
View original content to download multimedia:https://www.prnewswire.com/news-releases/a-small-step-towards-a-big-mission-worlds-first-ultradx-received-first-clinical-approval-of-single-molecule-analyzer-in-china-302239416.html
SOURCE UltraDx Bio