Promising long-term safety and effectiveness results from PAD population with extremely complex SFA disease
Promising long-term safety and effectiveness results from PAD population with extremely complex SFA disease
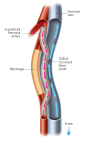
MILPITAS, Calif.--(BUSINESS WIRE)-- PQ Bypass, Inc, a medical device pioneer bringing new advancements to the treatment of complex peripheral artery disease (PAD), announces promising long-term safety and effectiveness results from 24-month follow-up data from the DETOUR1 Clinical Study, including a 79% primary patency, with 82% of patients maintaining freedom from major adverse events at 2 years post-procedure. The DETOUR1 trial is the first-in-man trial of the FDA-designated Breakthrough Device, The Detour System, evaluating the safety and effectiveness of the novel procedure and device system for treating long, complex lesions in the superficial femoral artery (SFA).
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20201113005130/en/
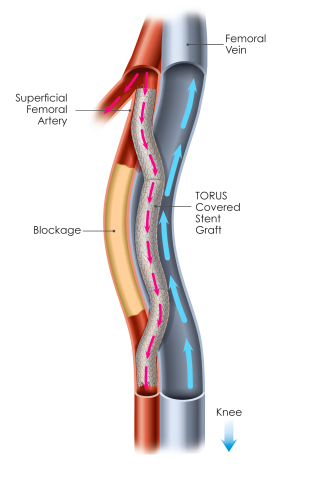
An illustration of a completed Detour Procedure (Graphic: Business Wire)
The data were released as one of the “10 Highly Anticipated Late-Breaking Clinical Trial Releases” during the VIVA 2020 Virtual Conference. The data release, titled “24-Month Outcomes from the DETOUR1 Trial for Percutaneous Femoropopliteal Bypass,” was presented by Ehrin Armstrong, MD, Professor of Medicine-Cardiology at the University of Colorado School of Medicine.
“The long-term safety and effectiveness results we are seeing coming out of the DETOUR1 trial really emphasize the potential for the Detour System as a viable treatment option for complex PAD in the SFA,” says Dr. Armstrong. “A 79% primary patency rate at 24 months post-procedure compares very favorably to the existing endovascular technologies that our practices are currently limited to, and the excellent safety results from such a complex patient cohort are extremely encouraging. The patient population for the DETOUR1 trial is fundamentally more advanced and complex than what is found in standard SFA trials, so I find these results nothing short of outstanding.”
Eight participating centers enrolled 81 limbs with complex, long-segment femoropopliteal lesions with moderate to severe calcification in lesions averaging 37cm across the trial and a 96% prevalence of CTO. All subjects have returned for their 24-month visit, with the following independently adjudicated endpoints:
– 79% primary patency
– 82% freedom from major adverse events, inclusive of death, clinically driven target lesion revascularization (CD-TLR), and target limb amputation
– 96% of subjects with maintained clinical success (Rutherford improvement of >1 class)
– 83% of subjects are clinically asymptomatic, with a Rutherford Class of 0
“We continue to be encouraged by the high-quality data and impressive results coming from the DETOUR1 Clinical Study,” says Heather Simonsen, President of PQ Bypass. “Now that the DETOUR2 IDE Trial has completed enrollment, we look forward to sharing additional data we collect on this novel device.”
About the Company:
PQ Bypass, Inc. is a rapidly advancing medical technology company pioneering a first-of-its-kind technology to address the complexity of treatment for severe peripheral arterial disease (PAD). Its proprietary Detour platform for percutaneous femoropopliteal bypass–designated by the FDA as a Breakthrough Device–is designed to be a significant technological advancement enabling novel, transformational interventions in outpatient settings. PQ Bypass is a former Company-In-Residence at the renowned Fogarty Institute for Innovation and is operated by recognized leaders in the medical device industry, including veterans from Boston Scientific, Phillips, Medtronic, Abbott, and Johnson & Johnson.
PQ Bypass is currently sponsoring two multicenter IDE trials, DETOUR2 and TORUS2, focused on complex SFA disease.
PQ Bypass is recognized by MedTech Outlook magazine as one of the Top 10 Cardiovascular Companies of 2019 and earned Frost and Sullivan’s European Technology Innovation award in 2017. The Detour System received FDA Breakthrough Device Designation in October 2020. The Detour System and the TORUS Stent Graft are limited by federal law to investigational use only and are not available for sale.
For more information, please visit www.pqbypass.com
For more information on Detour2, please visit https://clinicaltrials.gov/ct2/show/NCT03119233
For more information on Torus2, please visit https://clinicaltrials.gov/ct2/show/NCT04130737
View source version on businesswire.com: https://www.businesswire.com/news/home/20201113005130/en/
Victoria Versprille
VVersprille@PQBypass.com
908-328-5787
Source: PQ Bypass, Inc.
View this news release and multimedia online at:
http://www.businesswire.com/news/home/20201113005130/en