Policy
An unnamed FDA official also told reporters that it would be good for Moderna to “show some humility” and admit that it didn’t follow the regulator’s recommendations in testing its mRNA flu vaccine.
FEATURED STORIES
Bristol Myers Squibb, GSK and Merck are contributing drug ingredients as part of their deals with the White House but are keeping many of the terms of their agreements private.
Some 200 rare disease therapies are at risk of losing eligibility for a pediatric priority review voucher, a recent analysis by the Rare Disease Company Coalition shows. That could mean $4 billion in missed revenue for already cash-strapped biotechs.
The FDA’s rare pediatric disease priority review voucher program missed reauthorization at the last minute in 2024; advocates have been fighting to get it back ever since.
Subscribe to BioPharm Executive
Market insights and trending stories for biopharma leaders, in your inbox every Wednesday
THE LATEST
Anti-aging organization SENS Research Foundation sacked Chief Science Officer Aubrey de Grey over concerns he has attempted to influence an investigation of sexual harassment claims filed against him.
Many expect the full approval will lead to vaccine mandates, as that was one of the obstacles to it for many organizations.
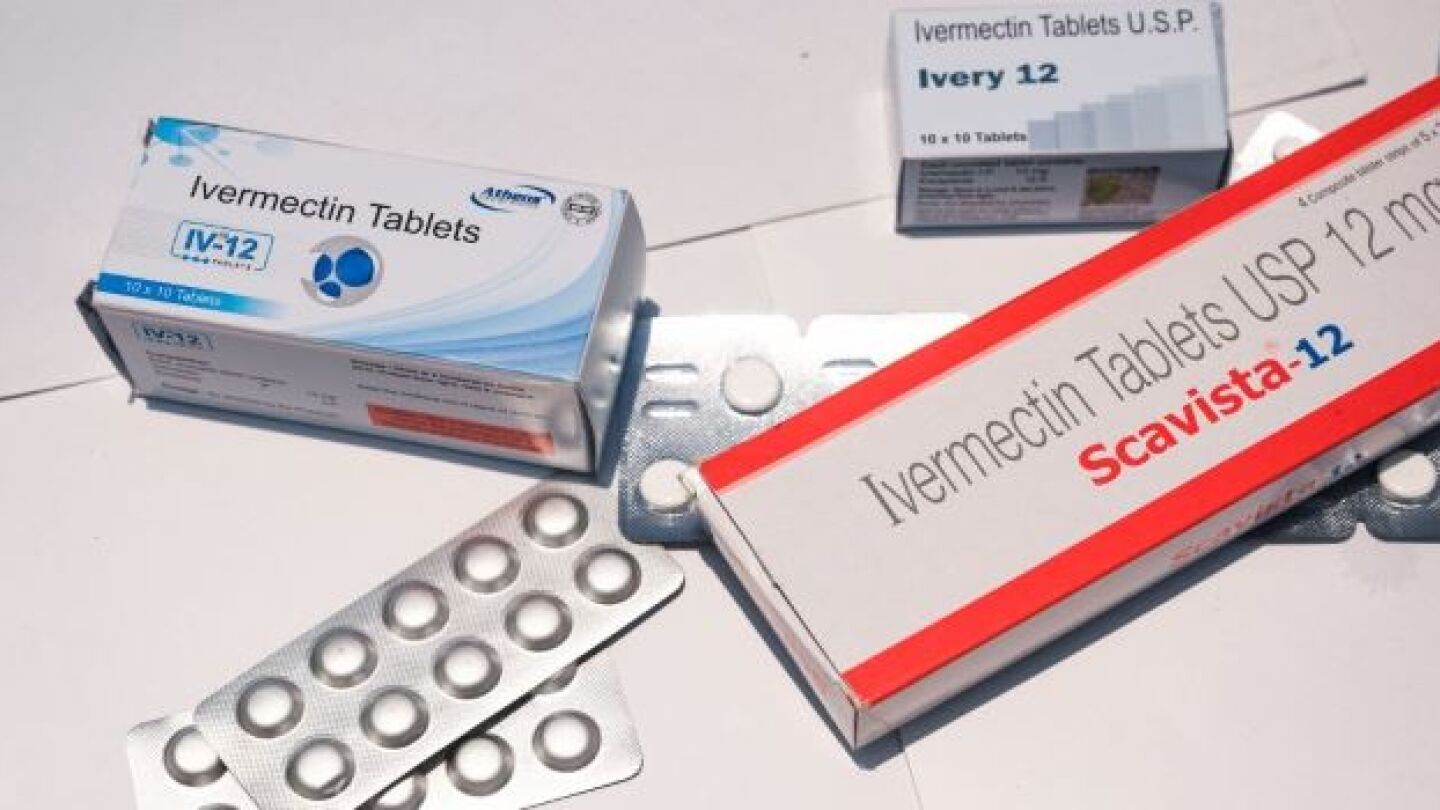
“You are not a horse. You are not a cow. Seriously, y’all. Stop it.” This is the FDA’s recently tweeted advice to Americans seeking out alternative, unapproved treatments for COVID-19.
The U.S. FDA has a busy schedule for the end of August and beginning of September. Let’s take a look.
Woodcock faces heavy opposition from an evenly divided U.S. Senate, particularly from West Virginia Sen. Joe Manchin, who has been critical of the approval process for Biogen’s Aduhelm, formerly known as aducanumab.
As governments and now even businesses push citizens to get vaccinated against COVID-19, anti-vaxxers are loading up more ammo for their argument with this week’s report.
The decision is largely based on three studies.
A Sackler family heir said in a court appearance that the family, which owns OxyContin drug maker Purdue Pharma, holds a “moral responsibility” to lend its help in combating the U.S. opioid crisis but noted it will not give billions of dollars to a legal settlement unless broad legal protections are provided in exchange.
Specifically, the new approval was for adults with dMMR recurrent or advanced solid tumors who have progressed on or after previous treatment and who have no satisfactory alternatives.
The latest biopharma insider trading scandal stems from Pfizer’s acquisition of Medivation in 2016.