Responding to an urgent need for medical ventilators for health professionals in Canada, StarFish Medical – Canada’s leading medical device design and development firm – called on NSK to quickly deliver up to 7,500 Monocarrier™ linear actuator assemblies for use in their Winnipeg 2.0 Ventilator.
ANN ARBOR, Mich., Nov. 16, 2020 /PRNewswire/ -- Responding to an urgent need for medical ventilators for health professionals in Canada, StarFish Medical – Canada's leading medical device design and development firm – called on NSK to quickly deliver up to 7,500 Monocarrier™ linear actuator assemblies for use in their Winnipeg 2.0 Ventilator.
Fast Response
In early Spring 2020, the Canadian government announced Canada's Plan to Mobilize Industry to fight COVID-19, authorizing the immediate production of ventilators by a group of companies led by StarFish Medical. Over the course of just 6 months, a redesign of the original Winnipeg Ventilator was developed, suppliers were selected, and government certification was granted.
According to Michael Stofferahn, Senior Vice President, NSK Americas, "The entire NSK Americas organization has responded with speed and precision in providing the engineering and manufacturing support that medical suppliers need in this period of crisis. That we can deliver for a company like StarFish Medical is testament to our rapid design and engineering capabilities, highly scalable production capacities, and our agile logistics platform."
Critical Application
The Winnipeg Ventilator 2.0 employs a revolutionary "frictionless" piston design. The piston is precision-machined so that its diameter is only a fraction of a millimeter smaller than the diameter of the cylinder in which it moves along a central axis. The absence of a physical seal or contact between piston and cylinder greatly reduces the resistance to piston motion, making it possible to obtain very high flow rates.
According to StarFish Medical EVP Strategic Relationships, John Walmsley, "To drive the piston, we chose NSK's Monocarrier for its smooth, accurate positioning and extreme durability – delivered in a small form factor – which is precisely what this new ventilator design required. NSK was our first choice for this critical function."
StarFish presented its new ventilator design to expert review panels and received positive feedback, including from Dr. Magdy Younes, MD, the original Winnipeg Ventilator designer. Younes tested the updated version of his ventilator and called it "a masterpiece."
Manufacturing
StarFish Medical engaged Toronto-based Celestica, Inc. a leading electronics manufacturing services company, to co-ordinate the supply chain and its vendor network for manufacturing.
"As soon as we received the StarFish innovative and manufacturable design for the new Winnipeg Ventilator 2.0, we leveraged our engineering, supply chain, and certified manufacturing expertise to source critical parts and begin production of up to 7,500 units without delay," said Kevin Walsh, Vice President, Celestica. "These ventilators are essential to treating critically-ill COVID-19 patients, so speeding time-to-market has never been more critical. We're proud to play a critical role in ensuring that StarFish meets its commitment to supply its ventilators to hospitals throughout Canada."
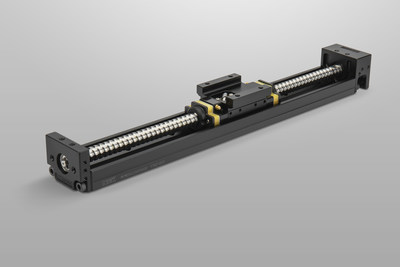
NSK Monocarrier™
The Monocarrier rodless linear actuator series combines three core NSK Automation technologies: precision-ground ball screws, linear guides with K1™ lubrication, and support bearings. These fully integrated components provide smooth, near-silent, high-accuracy linear positioning for up to 5 years or 10,000 km of travel without maintenance. They are available in a broad range of sizes and load capacities, from the very compact units used by StarFish Medical, to the Toughcarrier™ line of heavy-duty industrial units featuring cylindrical rollers as the rolling elements.
The Monocarrier actuator also takes advantage of NSK Automation's mechatronics expertise, and can be easily combined with stepper or servo motors for a seamlessly integrated, preassembled and pretested component ready for installation. Multiple Monocarriers can easily be combined into an XYZ assembly or integrated with NSK linear guides and rails using readily available combination brackets, to create high-precision multi-axis robots or gantry systems. For applications ranging from semiconductor manufacturing, medical and diagnostic equipment, to 3D printers and industrial machining systems, NSK enables automation to help customers pioneer new technology, increase operational efficiency, or go to market faster.
About NSK
NSK manufactured the world's first bearings in Japan in 1916, and has since developed into a global organization researching, designing, and manufacturing Motion & Control™ solutions essential for mobility and industrial applications. In the early 1960s, NSK set its sights outside Japan and has established over 200 business locations in 30 countries, alongside a vast network of joint ventures and partnerships in all corners of the world. Today, NSK is the top supplier of bearings in Japan and is the third largest supplier in the world by market share.
For more information, visit NSKAutomation.com
View original content to download multimedia:http://www.prnewswire.com/news-releases/nsk-monocarrier-accelerates-certification-of-ventilators-for-covid-19-301172314.html
SOURCE NSK Americas
Company Codes: Tokyo:6471, OTC-PINK:NPSKY