News
The current state of political affairs in the U.S. does not bode well for the direction of that turn. The country is at real risk of losing its long-held lead in biotech innovation.
FEATURED STORIES
The FDA’s refusal to review Moderna’s mRNA-based flu vaccine is part of a larger communications crisis unfolding at the agency over the past nine months that has also ensnarled Sarepta, Capricor, uniQure and many more.
The rare disease drugmaker is facing potential competitors for achondroplasia drug Voxzogo. Is a big M&A deal with two approved assets enough to maintain investor interest?
The FDA issued a rare Refusal-to-File letter to Moderna over its mRNA-based influenza vaccine application, in an unusual move that sent the biotech’s shares tumbling.
FROM OUR EDITORS
Read our takes on the biggest stories happening in the industry.
Novo Nordisk and Eli Lilly have been battling head-to-head in an exploding obesity market. They should never have been compared apples to apples.
THE LATEST
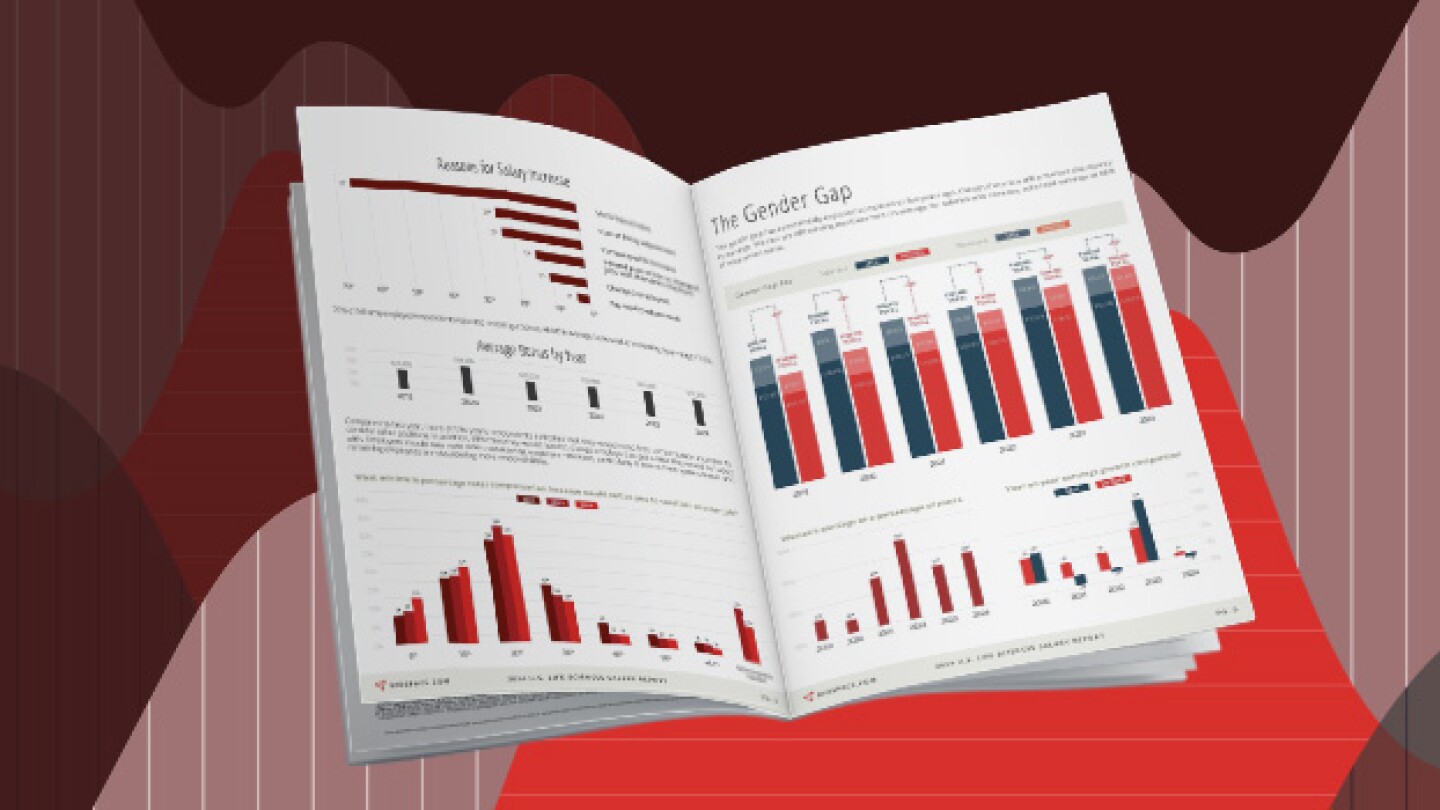
BioSpace’s 2024 Salary Report explores the average salaries and salary trends of life sciences professionals.
AstraZeneca reported Monday that adding Lynparza to Imfinzi improved outcomes in mismatch repair proficient endometrial cancer, more than doubling the median duration of response in patients.
With Boehringer Ingelheim’s announcement earlier this month that it was capping U.S. inhaler costs at $35 per month, AstraZeneca on Monday followed suit.
New late-stage trial results for GSK’s Jemperli show improved overall and progression-free survival in a broader range of endometrial cancer patients, which could lead to a potential label expansion.
Contineum Therapeutics joined the 2024 initial public offering class on Friday with an SEC filing. The biotech will use the IPO proceeds to complete a Phase II trial for its most mature candidate targeting multiple sclerosis.
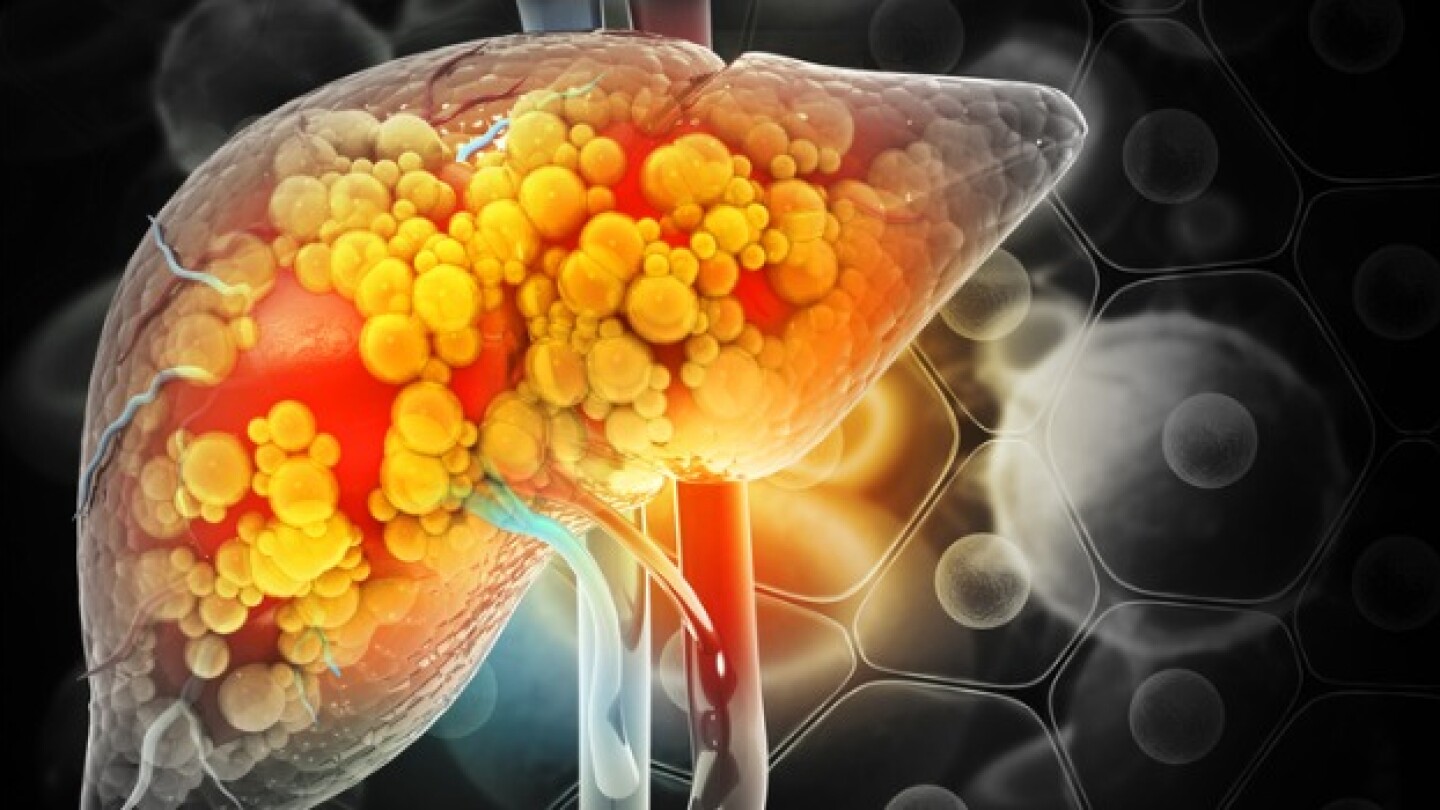
With its FDA approval last week and first-to-market advantage, Madrigal Pharmaceuticals’ Rezdiffra will set the standard for other metabolic dysfunction-associated steatohepatitis candidates in development.
By votes of 11-0 and 8-3, respectively, an FDA advisory committee Friday deemed the risks of early death for both Johnson & Johnson’s Carvykti and Bristol Myers Squibb’s Abecma acceptable.
Asgard Therapeutics, a Swedish gene therapy biotech, has closed a $32 million Series A round with help from prominent pharma players as it prepares for a 2026 IND.
Bayer will co-create a novel target identification platform that leverages Aignostics’ artificial intelligence technology and proprietary multimodal patient cohorts.
The Chinese biotechs are broadening their collaboration. Hansoh Pharma is licensing Biotheus’ anti-EGFR/cMet bispecific antibody to develop antibody-drug conjugates.