A new clinical study shows that Natera’s Signatera™ test identified colorectal cancer recurrence up to 16.5 months earlier than radiologic imaging by detecting traces of tumor DNA in the blood after surgery.
|
SAN CARLOS, Calif., May 9, 2019 /PRNewswire/ -- A new clinical study shows that Natera’s Signatera™ test identified colorectal cancer recurrence up to 16.5 months earlier than radiologic imaging by detecting traces of tumor DNA in the blood after surgery. The test also identified patients most likely to relapse, both before and after chemotherapy.1 Results were published in the May issue of JAMA Oncology.
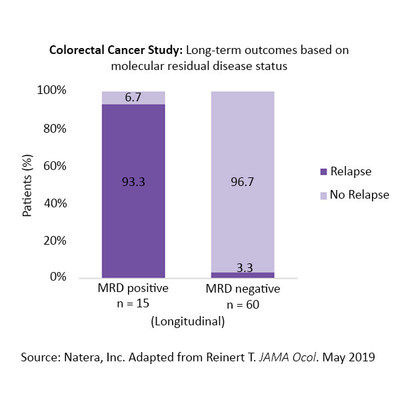
The prospective, multicenter study enrolled 130 patients with stage I–III colorectal cancer from Aarhus University, Randers, and Herning hospitals in Denmark. The study used Natera's Signatera research-use-only test to evaluate molecular residual disease (MRD) in 829 blood samples collected serially throughout the patient monitoring period. Results demonstrated that the Signatera test detected molecular recurrence up to 16.5 months earlier than standard-of-care radiologic imaging (average 8.7 months). Serial testing picked up 14 out of 16 relapses (patient-level sensitivity 88 percent), and among patients who did not relapse, 455 out of 456 post-surgical blood samples correctly tested negative (test-level specificity 99.8 percent). The study also found that MRD status was the most significant predictor of relapse after adjusting for all other known risk factors, including disease stage and lymph node status. Signatera MRD-positive patients who did not receive treatment relapsed in 93 percent of cases. Among patients who remained MRD-negative, the relapse rate was 3 percent. These results underscore the potential of MRD status to risk stratify patients more accurately after surgery to determine which patients need additional therapeutic interventions and which could be safely monitored. "Our study showed unequivocally that Natera's personalized multiplex PCR-based next-generation sequencing is a highly sensitive approach for detecting molecular residual disease in the blood," said Claus Lindbjerg Andersen, M.Sc., Ph.D., study lead investigator, Aarhus University. "The results show the potential of blood-based MRD detection to drive a paradigm-shift in how patients are managed during the course of their disease." "This study highlights Signatera's potential to change post-operative management of colorectal cancer," said Alexey Aleshin, M.D., MBA, Natera's oncology medical director. "We look forward to making this test available for clinical practice and pharmaceutical drug trials." With 1.3 million newly diagnosed cases each year worldwide, colorectal cancer is the third most common cancer and the second leading cause of cancer-related deaths.2 Despite implementation of screening and advances in treatment regimens, the five-year mortality rate remains high at about 40 percent.2-4 The study also reported the first published demonstration of Natera's plasma-based whole exome sequencing capability, in which there was strong concordance between whole exome results from the plasma and tumor biopsy at time of metastasis. The study, titled Analysis of Plasma Cell-Free DNA by Ultradeep Sequencing in Patients With Stages I to III Colorectal Cancer, can be found here. About Signatera™ The body of evidence on the utility of Signatera is growing, with multiple studies demonstrating the Signatera RUO method's ability to detect molecular residual disease, measure treatment response, and identify recurrence months or years earlier than the standard of care for a variety of cancer types, including breast cancer, early stage non-small cell lung cancer, bladder cancer, and colorectal cancer.1, 5-8 Based on numerous studies across multiple cancer types, a positive Signatera RUO result without further treatment has predicted clinical relapse over 98 percent of the time.1, 5-8 Natera will also offer a research-use-only service for plasma-based whole exome sequencing to create a personalized assay when tissue is not available, or reflexively for Signatera ctDNA positive cases, to characterize resistance mutations, actionable mutations, neoantigens, and tumor evolution. The service will interrogate approximately 20,000 genes from ctDNA to detect somatic mutations, representing a significant increase in coverage over most commercially available fixed liquid biopsy panels. If ordered as a combined service, researchers can first use Signatera to monitor patients for the presence or absence of ctDNA, and for positive patients they can reflex to a plasma exome to characterize tumor evolution using the same exact DNA library sample. Natera expects the service to become available in the second half of 2019. About Natera Forward-Looking Statements This test was developed by Natera, Inc. a laboratory certified under the Clinical Laboratory Improvement Amendments (CLIA). This test has not been cleared or approved by the U.S. Food and Drug Administration (FDA). Although FDA does not currently clear or approve laboratory-developed tests in the U.S., certification of the laboratory is required under CLIA to ensure the quality and validity of the tests. Contacts References
SOURCE Natera |
||
Company Codes: NASDAQ-NMS:NTRA |