Neurocrine Biosciences, Inc. (Nasdaq: NBIX), today announced that the complete study results from its Phase 3 KINECT™-HD study investigating valbenazine for the treatment of chorea associated with Huntington’s disease (HD) has been published in The Lancet Neurology online edition and will appear in the June 2023 print issue.
|
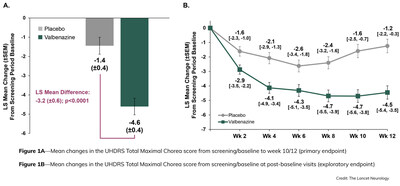
- Statistically Significant Improvement in Chorea Associated with Huntington's Disease Seen as Early as Week 2 - Chorea Improvement Supported by Statistically Significant Clinical Global Impression of Change (CGI-C) Response Status and Patient Global Impression of Change (PGI-C) Response Status Scores at Week 12 - First Phase 3 Study to Implement Huntington's Disease Health Index (HD-HI), a Patient-Reported Outcome Measure, Showed Reduced Disease Burden as Reported by Patients Receiving Valbenazine versus Placebo - Supplemental New Drug Application (sNDA) Filed, with Prescription Drug User Fee Act (PDUFA) Target Date Set for August 20, 2023 SAN DIEGO, May 19, 2023 /PRNewswire/ -- Neurocrine Biosciences, Inc. (Nasdaq: NBIX), today announced that the complete study results from its Phase 3 KINECT™-HD study investigating valbenazine for the treatment of chorea associated with Huntington's disease (HD) has been published in The Lancet Neurology online edition and will appear in the June 2023 print issue. The study met pre-defined primary and secondary endpoints of improvement in chorea severity and global impression of change, demonstrating a reduction in chorea symptoms associated with HD and improvement of overall chorea severity as noticed by clinicians and patients, with improvement seen as early as Week 2 of the initial dose in the 12-week study. "We're pleased to share the KINECT-HD full study results with the scientific community and its acceptance in an esteemed journal following rigorous peer-review," said Eiry W. Roberts, M.D., Chief Medical Officer at Neurocrine Biosciences. "There remains a need for symptomatic treatments for chorea associated with Huntington's disease, and this manuscript provides an in-depth overview of the KINECT-HD study data and the potential of valbenazine to fulfill this need." Valbenazine is a novel vesicular monoamine transporter 2 (VMAT2) inhibitor. VMAT2 inhibitors have been shown to reduce chorea associated with HD. "More than 90 percent of adults with HD experience chorea," said Erin Furr Stimming, M.D., the paper's lead author and professor in the Department of Neurology and Memorial Hermann Chair with McGovern Medical School at UTHealth Houston. "The KINECT-HD study data demonstrated statistically significant improvement in chorea associated with HD as compared with placebo. The safety profile for participants with chorea associated with HD was consistent with the known safety profile of valbenazine. By the end of the 12-week study, 82 percent of valbenazine-treated participants were taking 80 mg." The KINECT-HD randomized double-blind, placebo-controlled Phase 3 study was conducted with 128 participants at 46 Huntington Study Group (HSG)–credentialed sites in North America. The primary endpoint was a reduction in severity of chorea, the cardinal motor feature in HD, as measured by change in the Unified Huntington's Disease Rating Scale (UHDRS®) Total Maximal Chorea (TMC) score from baseline to the average score at weeks 10 and 12. The TMC score is part of the motor assessment of the UHDRS that measures chorea. In the KINECT-HD study, treatment with valbenazine resulted in a placebo-adjusted mean reduction in the TMC score of 3.2 units (P < 0.0001), indicating a highly statistically significant improvement in chorea (Figure 1A). Improvement was seen at Week 2 as participants completed the lowest study dose (40 mg), with consistently greater improvement relative to placebo in all subsequent visits (Weeks 4 to 12), as the dose was adjusted from 40 mg to 60 mg and 80 mg over the course of the 12-week study (Figure 1B). Secondary endpoints of Clinical Global Impression of Change (CGI-C) response status and Patient Global Impression of Change (PGI-C) response status were also statistically significant and supported the improvements in TMC score that were seen over the 12-week study period. At Week 12, more participants were classified as "much improved" or "very much improved" according to these key secondary endpoints when treated with valbenazine than placebo (CGI-C: 43 percent versus 13 percent; PGI-C: 53 percent versus 26 percent). No statistical difference between treatment groups was found in either of the two secondary Quality of Life in Neurological Disorders (Neuro-QoL) endpoints. Treatment-emergent adverse events, including somnolence, fatigue, fall and akathisia, were mild to moderate and consistent with the known safety profile of valbenazine. No suicidal behavior or worsening of suicidal ideation was observed in the valbenazine-treated subjects in this study. The KINECT-HD study marked the first-ever implementation of the Huntington's Disease Health Index (HD-HI) in a Phase 3 trial. HD-HI is a novel, validated, disease-specific, patient-reported outcome measure designed to evaluate clinically meaningful changes in HD function in response to therapeutic interventions. The HD-HI study results of these exploratory endpoints demonstrate improvement in mobility and hand/arm function and decreased burden from abnormal movements, as reported by patients receiving valbenazine compared to placebo. The U.S. Food and Drug Administration (FDA) accepted the supplemental New Drug Application for valbenazine as a treatment for chorea associated with HD in December 2022 and has set a Prescription Drug User Fee Act target action date of August 20, 2023, by which it will respond to the submission. The filing included data from the KINECT-HD Phase 3 study and the ongoing KINECT-HD2 open-label study of valbenazine in adults with chorea associated with HD. HD is a hereditary, progressive, and ultimately fatal neurodegenerative disorder in which the loss of certain neurons within the brain causes motor, cognitive and psychiatric symptoms. More than nine out of 10 people with HD will experience chorea, an involuntary movement disorder, at some point in the course of their disease. HD chorea has been shown to significantly impact physical, social and emotional function among those living with HD. About the KINECT™-HD Study Top-line clinical data from the KINECT-HD study were reported in December 2021 and demonstrated that adults with chorea associated with HD had a significantly greater improvement in the Unified Huntington's Disease Rating Scale (UHDRS®) Total Maximal Chorea (TMC) score when treated with valbenazine versus placebo (least squares mean difference of -3.2; 95 percent confidence interval, -4.4 to -2.0; P < 0.0001). The secondary endpoints included Clinical Global Impression of Change (CGI-C) response status and Patient Global Impression of Change (PGI-C) response status for valbenazine treatment. The treatment-emergent adverse events in this study were consistent with the known safety profile of valbenazine. No suicidal behavior or worsening of suicidal ideation were observed in the valbenazine-treated subjects in this study. KINECT-HD and KINECT-HD2 clinical study data were included in the supplemental new drug application (sNDA), which was accepted by the U.S. Food and Drug Administration (FDA) in December 2022, with a Prescription Drug User Fee Act (PDUFA) target action date of August 20, 2023, by which it will respond to the submission. For more information on this KINECT-HD study, please visit www.huntingtonstudygroup.org. About KINECT™-HD2 About Huntington's Disease Health Index (HD-HI) About Chorea Associated with Huntington's Disease (HD) About Huntington Study Group / HSG Clinical Research, Inc. The KINECT-HD study was conducted in cooperation with the HSG and the Clinical Trials Coordination Center (CTCC) at the University of Rochester Medical Center's Center for Health + Technology (CHeT). For more information, visit the CTCC website https://www.urmc.rochester.edu/health-technology/our-expertise/clinical-trials-coordination.aspx. About Neurocrine Biosciences, Inc. Neurocrine and the Neurocrine logo are registered trademarks, and KINECT is a trademark of Neurocrine Biosciences, Inc. Neurocrine Biosciences Forward-Looking Statements
SOURCE Neurocrine Biosciences, Inc. |
||
Company Codes: NASDAQ-NMS:NBIX |