Insulet Corporation, the global leader in tubeless insulin pump technology with its Omnipod® brand of products, announced it has received clearance from the U.S. Food and Drug Administration for its Omnipod® 5 Automated Insulin Delivery System for individuals aged six years and older with type 1 diabetes.

ACTON, Mass.--(BUSINESS WIRE)-- Insulet Corporation (NASDAQ: PODD) (Insulet or the Company), the global leader in tubeless insulin pump technology with its Omnipod® brand of products, today announced it has received clearance from the U.S. Food and Drug Administration (FDA) for its Omnipod® 5 Automated Insulin Delivery System (Omnipod 5) for individuals aged six years and older with type 1 diabetes. Omnipod 5 is the first tubeless automated insulin delivery (AID) system that integrates with the Dexcom G6 Continuous Glucose Monitoring (CGM) System and a compatible smartphone to automatically adjust insulin and help protect against highs and lows.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20220127006047/en/
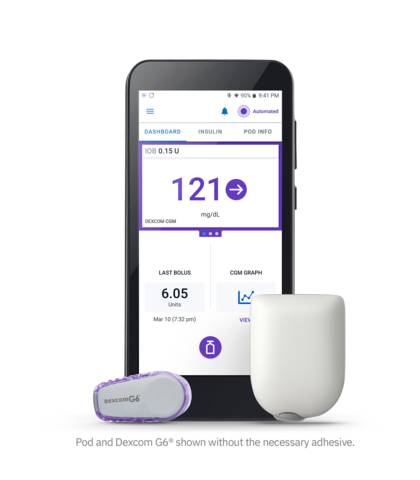
Omnipod® 5 Automated Insulin Delivery System (Photo: Business Wire)
Omnipod 5 is designed to make it easier than ever to manage glucose with no multiple daily injections, no tubes, and zero fingersticks1 to help simplify life with diabetes.
“Omnipod 5 is a life-changing technology that we believe will revolutionize the market and the lives of people with diabetes,” said Shacey Petrovic, President and Chief Executive Officer of Insulet. “We are incredibly proud of this simple-to-use, elegant system, designed to deliver unmatched freedom and to greatly simplify insulin management and improve glucose control for our users.”
The Omnipod 5 System2 consists of the tubeless Pod enhanced with SmartAdjust™ technology, the Dexcom G6 CGM, and the Omnipod 5 mobile app with its integrated SmartBolus Calculator. The user has the option to download this app onto a compatible personal smartphone or to use the Omnipod 5 Controller, which is provided free with the first prescription.
Every five minutes, SmartAdjust receives a Dexcom CGM value and trend, and predicts where glucose will be 60 minutes into the future. The system then increases, decreases, or pauses insulin delivery using the user’s desired and customized glucose target, helping to protect against highs and lows.
“As the pioneer of integrated CGM, we are excited to see our years of collaborative work with Insulet culminate into the first and only FDA-cleared tubeless automated insulin delivery system,” said Kevin Sayer, Chairman, President and CEO of Dexcom. “Omnipod 5 combines the accuracy and unmatched user experience of the Dexcom G6 CGM with the simplicity of tubeless insulin delivery to offer people with diabetes a revolutionary new way to optimize time in range.”
Omnipod 5 will launch through the pharmacy channel, providing customer benefits of no contract, no commitment, and no obligation. In the coming days, Insulet will offer Omnipod 5 in a limited market release so that the Company can incorporate learnings to deliver a best-in-class product experience. Omnipod 5 is expected to be broadly available shortly after the limited market release.
Omnipod 5 was recently selected as a CES® 2022 Innovation Award honoree in two categories: Health and Wellness and Wearable Technologies. The CES Innovation Awards is an annual competition honoring outstanding design and engineering in consumer technology products.
People with diabetes can enjoy the benefits of Pod Therapy today through OmnipodPromise™, which allows new and existing users to start on Omnipod DASH® and upgrade to Omnipod 5 at no additional cost when coverage is available.
To learn more, visit the Omnipod website.
Insulet will provide additional details about today’s announcement on the Company’s fourth quarter 2021 earnings conference call on February 23, 2022 at 4:30 p.m. (Eastern Time). The link to the live call will be available here and will be archived for future replay. To participate in the live call via phone, please pre-register online here to receive a telephone number, passcode, and a unique registrant ID required to enter the call.
About Insulet Corporation:
Insulet Corporation (NASDAQ: PODD), headquartered in Massachusetts, is an innovative medical device company dedicated to simplifying life for people with diabetes and other conditions through its Omnipod product platform. The Omnipod Insulin Management System provides a unique alternative to traditional insulin delivery methods. With its simple, wearable design, the disposable Pod provides up to three days of non-stop insulin delivery, without the need to see or handle a needle. Insulet also leverages the unique design of its Pod by tailoring its Omnipod technology platform for the delivery of non-insulin subcutaneous drugs across other therapeutic areas. For more information, please visit insulet.com and omnipod.com.
Forward-Looking Statement:
This press release may contain forward-looking statements concerning Insulet's expectations, anticipations, intentions, beliefs, or strategies regarding the future. These forward-looking statements are based on its current expectations and beliefs concerning future developments and their potential effects on Insulet. There can be no assurance that future developments affecting Insulet will be those that it has anticipated. These forward-looking statements involve a number of risks, uncertainties (some of which are beyond its control) or other assumptions that may cause actual results or performance to be materially different from those expressed or implied by these forward-looking statements, and other risks and uncertainties described in its Annual Report on Form 10-K, which was filed with the Securities and Exchange Commission on February 24, 2021 in the section entitled "Risk Factors," and in its other filings from time to time with the Securities and Exchange Commission. Should one or more of these risks or uncertainties materialize, or should any of its assumptions prove incorrect, actual results may vary in material respects from those projected in these forward-looking statements. Insulet undertakes no obligation to publicly update or revise any forward-looking statements.
1 If a user’s glucose alerts and readings from the G6 do not match symptoms or expectations or a user is taking over the recommended maximum dosage amount of 1000mg of acetaminophen every six hours, one should use a blood glucose meter to make diabetes treatment decisions.
2 The Omnipod 5 Automated Insulin Delivery System is comprised of SmartAdjust™ technology (cleared in K203774), the Omnipod 5 Pod and Omnipod 5 App (cleared in K203768) and the Omnipod 5 SmartBolus Calculator (cleared in K203772). Integration with the Dexcom G6 CGM is required for automated insulin delivery.
©2022 Insulet Corporation. Omnipod, Omnipod DASH, Omnipod 5, SmartAdjust, and OmnipodPromise are trademarks or registered trademarks of Insulet Corporation in the United States of America and other various jurisdictions. All rights reserved. Dexcom G6 is a registered trademark of Dexcom and used with permission. All other trademarks are the property of their respective owners. The use of third-party trademarks does not constitute an endorsement or imply a relationship or other affiliation.
View source version on businesswire.com: https://www.businesswire.com/news/home/20220127006047/en/
Investor Relations:
Deborah R. Gordon
Vice President, Investor Relations
(978) 600-7717
dgordon@insulet.com
Media:
Angela Geryak Wiczek
Senior Director, Corporate Communications
(978) 932-0611
awiczek@insulet.com
Source: Insulet Corporation
View this news release and multimedia online at:
http://www.businesswire.com/news/home/20220127006047/en






