PHM announces the publication of a case report summarizing a patient’s complete response to an innovative treatment for prostate cancer.
PHM case report reinforces importance of access to treatments
LOS ANGELES, July 11, 2023 /PRNewswire/ -- PHM announces the publication of a case report summarizing a patient's complete response to an innovative treatment for prostate cancer. PHM is a clinically and scientifically sophisticated healthcare navigation company that solves complex healthcare problems to help individuals get well and stay well.
Despite decades of research and multiple treatment options, prostate cancer remains the second leading cause of cancer death in men in the U.S. according to the American Cancer Society, with a new case being diagnosed every three minutes, per the Prostate Cancer Foundation. PHM's peer-reviewed case report, recently accepted in Frontiers in Oncology, highlights the importance of having access to cutting-edge cancer diagnostics and therapeutics.
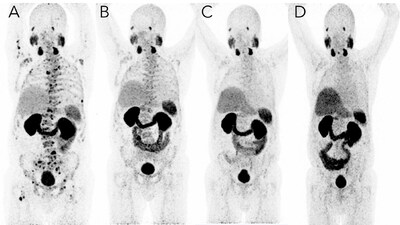
The report summarizes the case of a 73-year-old male diagnosed with stage 4B prostate cancer, which has a five-year survival rate of ~30%. Despite standard chemo and hormonal therapies, his cancer continued to worsen. After only four treatments of a state-of-the-art, targeted radioligand therapy while continuing hormone therapy, scans showed that the disease had disappeared. Today, five years later, the patient remains disease-free.
"The patient came to PHM about a year after he was diagnosed, and there were early signs that standard treatments were not going to adequately control his disease. We connected him to a clinic in Germany employing theranostics, a treatment for prostate cancer that could potentially help but was not yet approved in the U.S.," said Eva Gordon, PhD, SVP Research and Chief Scientist at PHM. "Access to treatments like these can improve both survival and quality of life for patients. We're proud we could help this patient access an emerging treatment at a time his prognosis was poor, and his options were otherwise limited."
In 2022, 5 years after the patient received his first treatment, the U.S. FDA approved this treatment. "This case is a testament to the remarkable impact this treatment can have for metastatic prostate cancer patients. I'm thrilled more patients can now benefit from theranostics," says treating physician, Richard Baum, MD, PhD., Head of Theranostic Center at Curanosticum Wiesbaden/Frankfurt, Professor of Nuclear Medicine at University of Frankfurt/Main.
The patient shares his perspective: "I went to see an oncologist and he told me I didn't have much time, maybe six months to one year if I went to chemotherapy. My PHM team told me about a new treatment available in Germany. After the first treatment the doctor showed me the scans and said the cancer had retreated a bit and he was encouraged. It got smaller with each subsequent treatment, and after the fourth treatment he couldn't see any remaining disease."
About PHM
PHM solves complex healthcare problems to help individuals get well and stay well. The company applies its deep health intelligence to bring the best of what's possible in medicine to individuals, families and companies that make their employees' health a priority. PrivateHealth.com.
![]() View original content to download multimedia:https://www.prnewswire.com/news-releases/innovative-treatment-for-prostate-cancer-shows-long-term-success-301873565.html
View original content to download multimedia:https://www.prnewswire.com/news-releases/innovative-treatment-for-prostate-cancer-shows-long-term-success-301873565.html
SOURCE Private Health Management