SCOTTSDALE, AZ--(Marketwired - October 29, 2013) - Hope Pharmaceuticals today announced that the United States Patent and Trademark Office issued U. S. patent no. 8,568,793, titled "Sodium Nitrite-Containing Pharmaceutical Compositions." The patent relates to the active pharmaceutical ingredient, its formulation and use in the company's Nithiodote and Sodium Nitrite Injection products. The patent is set to expire in December 2031, absent any extension.
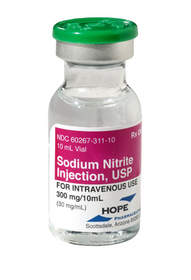
Hope Pharmaceuticals manufactures Sodium Nitrite Injection which is formulated with the new sodium nitrite material.
The United States Food and Drug Administration (FDA) approved Hope Pharmaceuticals' Sodium Nitrite Injection for sequential use with Sodium Thiosulfate Injection for the treatment of acute cyanide poisoning that is judged to be life-threatening. Sodium Nitrite Injection is co-packaged with Sodium Thiosulfate Injection in a kit called Nithiodote. It is also available as an individually-packaged medication. Prescribing information for Nithiodote and Sodium Nitrite Injection is available online at www.nithiodote.com and www.nitrite.com, respectively.
Hope Pharmaceuticals is currently the only supplier of Sodium Nitrite Injection in the United States.
Hope Pharmaceuticals is a privately owned company located in Scottsdale, Arizona.
Help employers find you! Check out all the jobs and post your resume.