Hancock Jaffe Laboratories, Inc., a developer of medical devices that restore cardiac and vascular health, announced that the ninth VenoValve patient in HJLI’s first-in-human, clinical study has successfully reached the one-year milestone.
Patient Continues to Show Significant Improvement at One Year
IRVINE, CA / ACCESSWIRE / October 21, 2020 / Hancock Jaffe Laboratories, Inc. (Nasdaq:HJLI, HJLIW), a developer of medical devices that restore cardiac and vascular health, announced today that the ninth VenoValve patient in HJLI's first-in-human, clinical study has successfully reached the one-year milestone. Patient 9's chronic venous insufficiency ("CVI") has dramatically improved when compared to pre-surgery levels, with reflux (the backwards flow of blood) improving 75%, disease manifestations as measured by a venous clinical severity scores ("VCSS") improving 73%, and pain, as measured on a visual analog scale ("VAS"), improving 100%.
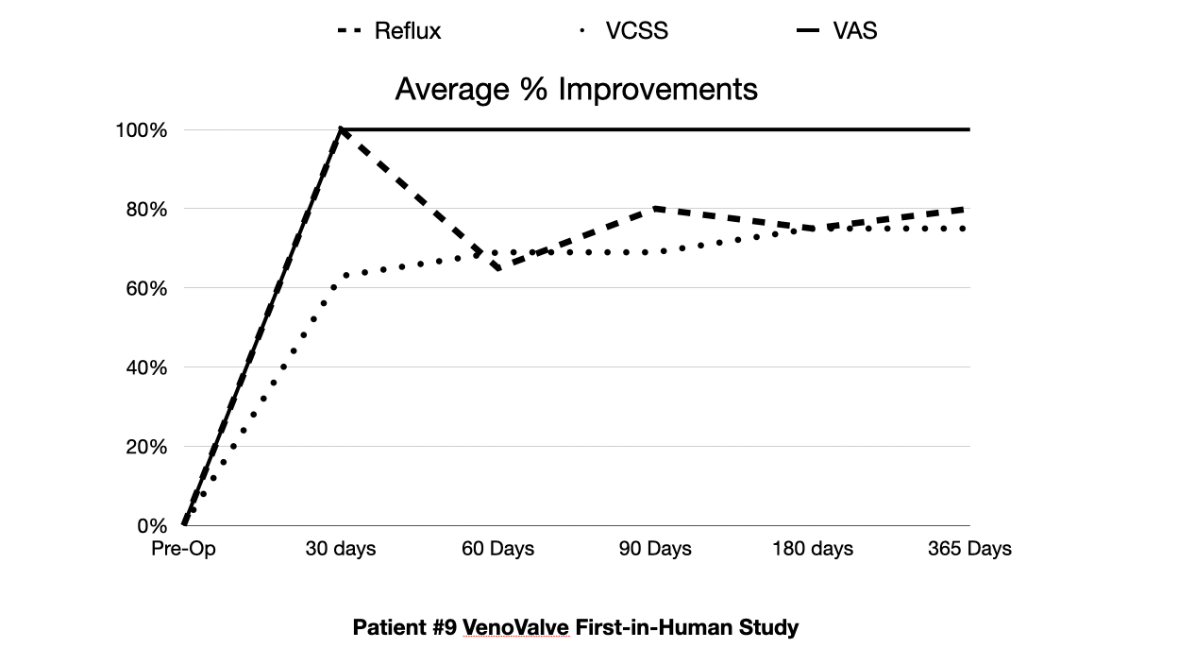
The patient's presurgery levels for reflux, VCSS, and VAS were 2, 16, and 5, respectively. At one year post surgery, those levels were 0.5, 4, and 0, respectively. The improvement in reflux is significant, as the patient now has reflux in the range of what you would expect in a normal patient, without CVI. The VCSS improvement of 12 points means that the patient went from having an active ulcer and severe CVI, to barely any visible signs of the disease. A VAS score of 0 means that the patient is now completely pain-free. Overall, nine VenoValve patients have now completed the one-year first-in-human trial.
Dr. Marc H. Glickman, Hancock Jaffe's Senior Vice President and Chief Medical Officer stated, "It is remarkable for a patient who had severe chronic venous insufficiency to be completely pain free one year after VenoValve surgery. Our goal was never perfection and was to achieve marginal improvement in the lives of patients who suffer from this debilitating disease and who have no other effective treatment options. Thus far, our results have far exceeded our expectations."
CVI occurs when the valves in the veins of the leg are injured or destroyed, causing blood to flow backwards, which is known as reflux. Reflux results in increased venous pressure (venous hypertension), damage to the veins, and results in the pooling of blood in the lower leg. Deep venous CVI is a serious condition, often resulting in debilitating pain, swelling, and open sores (venous ulcers) on the lower leg. HJLI has implanted VenoValves in 11 patients over the course of a year as part of its first-in-man, Colombian study, which is the pre-cursor to the U.S. pivotal trial. Data is being reported today for the first eight VenoValve patients to reach the critical, one-year milestone. The eight VenoValve patients that are one-year post surgery have now completed the first-in-man, clinical study and this phase of the VenoValve study will conclude in December when the three remaining patients are all one year post surgery.
Next steps for the VenoValve include the continued monitoring of the remaining two VenoValve patients in Colombia, a Pre-IDE filing with the U.S. Food and Drug Administration ("FDA"), the completion of a series of functional tests and a GLP study mandated by the FDA, and the filing of an IDE application with the FDA, seeking approval to begin the U.S. pivotal trial, which HJLI expects to file in the first quarter of 2021.
Approximately 2.4 million people in the U.S. suffer from CVI due to reflux in the deep venous system. Estimates indicate that direct medical costs from CVI in the U.S. exceed $38 Billion a year. There are currently no FDA approved devices, or effective treatments for deep venous CVI.
About Hancock Jaffe Laboratories, Inc.
Hancock Jaffe Laboratories (NASDAQ:HJLI) specializes in developing and manufacturing bioprosthetic (tissue based) medical devices to establish improved standards of care for treating cardiac and vascular diseases. Hancock Jaffe currently has two lead product candidates: the VenoValve®, a porcine based valve which is intended to be surgically implanted in the deep venous system of the leg to treat reflux associated with Chronic Venous Insufficiency; and the CoreoGraft®, a bovine tissue based off the shelf conduit intended to be used for coronary artery bypass surgery. Hancock Jaffe has a 20-year history of developing and producing FDA approved medical devices that sustain or support life. The current management team at Hancock Jaffe has been associated with over 50 FDA or CE marked medical devices. For more information, please visit HancockJaffe.com.
Cautionary Note on Forward-Looking Statements
This press release and any statements of stockholders, directors, employees, representatives and partners of Hancock Jaffe Laboratories, Inc. (the "Company") related thereto contain, or may contain, among other things, certain "forward-looking statements" within the meaning of the Private Securities Litigation Reform Act of 1995. Such forward-looking statements involve significant risks and uncertainties. Such statements may include, without limitation, statements identified by words such as "projects," "may," "will," "could," "would," "should," "believes," "expects," "anticipates," "estimates," "intends," "plans," "potential" or similar expressions. These statements are based upon the current beliefs and expectations of the Company's management and are subject to significant risks and uncertainties, including those detailed in the Company's filings with the Securities and Exchange Commission. Actual results (including, without limitation, the performance of the new board members described herein) may differ significantly from those set forth or implied in the forward-looking statements. These forward-looking statements involve certain risks and uncertainties that are subject to change based on various factors (many of which are beyond the Company's control). The Company undertakes no obligation to publicly update any forward-looking statements, whether as a result of new information, future presentations or otherwise, except as required by applicable law.
SOURCE: Hancock Jaffe Laboratories, Inc.
View source version on accesswire.com:
https://www.accesswire.com/611569/Hancock-Jaffe-Announces-One-Year-Follow-up-Data-on-Ninth-VenoValve-Patient




