Mean best-corrected visual acuity (BCVA) among GS010-treated eyes and sham-treated eyes evolved with similar trajectories, worsening to a low point, or nadir, before beginning to improve by Week 48 – coherent with REVERSE trial
- Mean best-corrected visual acuity (BCVA) among GS010-treated eyes and sham-treated eyes evolved with similar trajectories, worsening to a low point, or nadir, before beginning to improve by Week 48 – coherent with REVERSE trial
- Statistical analysis suggests that at Week 48, GS010-treated eyes were three times more likely than sham-treated eyes to have a level of vision of 20/200 or better, the threshold for legal blindness
- 1 in 4 subjects showed better BCVA from baseline in their GS010-treated eye than in their sham-treated eye (at least 0.3 LogMAR or 15 ETDRS letters, a clinically meaningful difference)
- 1 in 4 subjects showed better change in low-contrast sensitivity in their GS010-treated eye than in their sham-treated eye (at least 0.3 LogCS, a clinically meaningful difference)
- Anatomic measures showed results broadly consistent with the direction of BCVA evolution
- GS010 reported to be safe and well-tolerated
PARIS--(BUSINESS WIRE)-- Regulatory News:
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20190203005038/en/
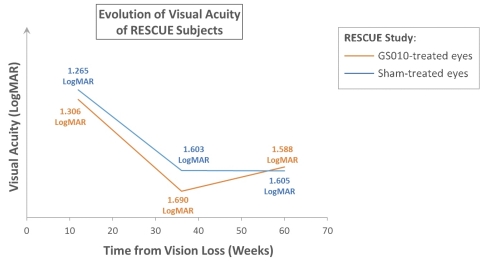
Illustration of the progression of visual acuity in RESCUE (Graphic: Business Wire)
GenSight Biologics (Paris: SIGHT) (Euronext: SIGHT, ISIN: FR0013183985, PEA-PME eligible), a biopharma company focused on discovering and developing innovative gene therapies for retinal neurodegenerative diseases and central nervous system disorders, today announced results from the first scheduled readout, at Week 48, of the RESCUE Phase III clinical trial evaluating the safety and efficacy of a single intravitreal injection of GS010 (rAAV2/2-ND4) in 39 subjects whose visual loss due to 11778-ND4 Leber Hereditary Optic Neuropathy (LHON) occurred up to 6 months prior to study treatment. These subjects received GS010 in one eye and a sham injection in the other eye, with drug treatment randomized between best- and worst-affected eyes.
Visual loss in LHON usually progresses such that vision reaches a nadir in 3 to 5 months, before stabilizing; the duration of this progression to nadir varies from patient to patient. In RESCUE, mean best-corrected visual acuity (BCVA) of GS010-treated eyes and sham-treated eyes evolved with similar trajectories, worsening to a low point before showing an improvement at Week 48. At Week 48, change from baseline for GS010-treated eyes was -19 ETDRS letters equivalent, while that for sham-treated eyes -20 ETDRS letters equivalent. These figures incorporate a recovery from the nadir of vision loss for drug- and sham-treated eyes: mean improvement over the nadir of vision loss was +13 ETDRS letters equivalent in GS010-treated eyes and +11 ETDRS letters equivalent in sham-treated eyes. The primary efficacy endpoint, defined as a +15-letter difference in visual acuity improvement for GS010-treated eyes compared to sham-treated eyes at 48 weeks, was not met.
Figure 1. Illustration of the progression of visual acuity in RESCUE
For illustrative purposes: mean observed baseline LogMAR values employed (no data imputation). Visual acuity changes over time. A positive slope, upward, on the vertical (Y) axis, represents improvement; a negative slope, downward, worsening of vision. As RESCUE subjects had vision loss for 0 to 6 months before treatment, assumed average time for cohort from visual loss to treatment plotted at 3 months (12 weeks).
Planned analysis of other visual functions and anatomic measures showed results broadly consistent with the direction of BCVA evolution: similar trajectories for GS010-treated and sham-treated eyes with the difference in change from baseline not being statistically significant at Week 48. The difference between GS010-treated and sham-treated eyes in change from baseline of temporal retinal nerve fiber layer missed statistical significance (p=0.0513). The changes from baseline in GS010-treated eyes of papillo-macular bundle thickness and ganglion cell volume were numerically superior to those in sham-treated eyes, though not statistically significant (p values > 0.05).
Even at an early readout at Week 48, some trends point toward GS010 efficacy. GS010-treated eyes were significantly more likely than sham-treated eyes to have 20/200 or better vision, the threshold for legal blindness (statistically significant with p=0.0347; odds ratio = 2.9). Subject responder analysis showed that in 24% of subjects, the change from baseline of high-contrast visual acuity in GS010-treated eyes was at least 0.3 LogMAR (15 ETDRS letters) better than in sham-treated eyes. Another subject responder analysis showed that in 24% of subjects, the change from baseline of low-contrast acuity (measured on the Pelli-Robson scale) in GS010-treated eyes was at least 0.3 LogCS better than in sham-treated eyes.
“The powerful and rapid degeneration of neurons early in the disease, combined with the time needed for GS010 to cause functioning proteins to be expressed, may be confounding efficacy measurements early in the active progression phase,” noted Dr. José-Alain Sahel, Director of the Institut de la Vision (Sorbonne-Université/Inserm/CNRS), Paris; Chairman of the Department of Ophthalmology at Centre Hospitalier National d'Ophtalmologie des XV-XX, Paris; Professor and Chairman of the Department of Ophthalmology at University of Pittsburgh School of Medicine and UPMC (University of Pittsburgh Medical Center); and co-founder of GenSight.
Dr. Barrett Katz, Chief Medical Officer of GenSight, added “In our REVERSE trial, which included patients with vision loss between 6 and 12 months prior to treatment, we saw more improvement in both anatomic measures and visual functions as the disease entered its chronic phase. The planned readouts of RESCUE data at Weeks 72 and 96 should confirm GS010’s efficacy.”
Figure 2. Illustration of the coherence between RESCUE and REVERSE
For illustrative purposes: mean observed baseline LogMAR values employed (no data imputation). Visual acuity changes over time. A positive slope, upward, on the vertical (Y) axis, represents improvement; a negative slope, downward, worsening of vision. As RESCUE subjects had vision loss for 0 to 6 months before treatment, assumed average time for cohort from visual loss to treatment plotted at 3 months (12 weeks). In REVERSE, subjects had vision loss of 6 to 12 months before treatment; assumed average time for cohort from visual loss to treatment plotted at 9 months (36 weeks).
“As expected, the RESCUE results at Week 48 present a more complex picture because of the intense, brutal and extremely rapid onset of the retinal ganglion cells’ degeneration.” said Bernard Gilly, Co-founder and Chief Executive Officer of GenSight. “But as with REVERSE, later readouts are likely to confirm our confidence in the efficacy of GS010. We eagerly await these upcoming readouts, even as we prepare to discuss the results with the relevant authorities. We remain committed to bringing GS010 as early as possible to the market so that LHON patients can have an effective treatment for their relentlessly blinding disease.”
Based on preliminary analysis of the safety data, GS010 was well-tolerated through 48 weeks. There were no serious ocular adverse events or discontinuations due to ocular issues. The most frequently seen ocular adverse events were related to the injection procedure itself. Transient elevations of intraocular pressure were occasionally seen but were thought secondary to intraocular inflammation and thought likely due to administration of GS010. Such episodes were without sequelae and responded to conventional treatment. There were no systemic serious adverse events or discontinuations related to study treatment or study procedure.
RESCUE subjects will be evaluated again at 72 and 96 weeks; results are expected to be available in Q2 (April) and Q3 2019, respectively. Week 96 data will be available in Q2 (May) 2019 for the REVERSE trial; its data will be unblinded at this evaluation, allowing subject-level analyses to be conducted.
The third interventional study for GS010, REFLECT, is a randomized, double-masked, placebo-controlled Phase III trial evaluating the safety and efficacy of bilateral injections of GS010 in patients up to one year from onset of vision loss due to LHON. The first patient in REFLECT was treated in March 2018.
The Company will host a conference call today, February 4, 2019, at 10am CET in French, and at 2.30pm CET (8.30am ET) in English, to discuss these results.
|
Webcast & Conference call in French |
| Dial-in numbers: |
| France Toll: +33170710159 PIN: 15229510# |
| United Kingdom Toll: +442071943759 PIN: 15229510# |
|
Webcast link: https://channel.royalcast.com/webcast/gensightbiologicsfr/20190204_1/ |
|
Webcast & Conference call in English |
| Dial-in numbers: |
| France Toll: +33 172727403 PIN: 53959568# |
| United Kingdom Toll: +442071943759 PIN: 53959568# |
| United States Toll: +1 6467224916 PIN: 53959568# |
|
Webcast link: https://channel.royalcast.com/webcast/gensightbiologicsen/20190204_1/ |
A replay of the calls and webcasts will be available by using the above links.
About GenSight Biologics
GenSight Biologics S.A. is a clinical-stage biopharma company focused on discovering and developing innovative gene therapies for retinal neurodegenerative diseases and central nervous system disorders. GenSight Biologics’ pipeline leverages two core technology platforms, the Mitochondrial Targeting Sequence (MTS) and optogenetics to help preserve or restore vision in patients suffering from blinding retinal diseases. GenSight Biologics’ lead product candidate, GS010, is in Phase III trials in Leber Hereditary Optic Neuropathy (LHON), a rare mitochondrial disease that leads to irreversible blindness in teens and young adults. Using its gene therapy-based approach, GenSight Biologics’ product candidates are designed to be administered in a single treatment to affected eyes by intravitreal injection to offer patients a sustainable functional visual recovery.
About GS010
GS010 targets Leber Hereditary Optic Neuropathy (LHON) by leveraging a mitochondrial targeting sequence (MTS) proprietary technology platform, arising from research conducted at the Institut de la Vision in Paris, which, when associated with the gene of interest, allows the platform to specifically address defects inside the mitochondria using an AAV vector (Adeno-Associated Virus). The gene of interest is transferred into the cell to be expressed and produces the functional protein, which will then be shuttled to the mitochondria through specific nucleotidic sequences in order to restore the missing or deficient mitochondrial function.
About Leber Hereditary Optic Neuropathy (LHON)
Leber Hereditary Optic Neuropathy (LHON) is a rare maternally inherited mitochondrial genetic disease, characterized by the degeneration of retinal ganglion cells that results in brutal and irreversible vision loss that can lead to legal blindness, and mainly affects adolescents and young adults. LHON is associated with painless, sudden loss of central vision in the 1st eye, with the 2nd eye sequentially impaired. It is a symmetric disease with poor functional visual recovery. 97% of patients have bilateral involvement at less than one year of onset of vision loss, and in 25% of cases, vision loss occurs in both eyes simultaneously. The estimated incidence of LHON is approximately 1,400 to 1,500 new patients who lose their sight every year in the United States and Europe.
About RESCUE and REVERSE
RESCUE and REVERSE are two separate randomized, double-masked, sham-controlled Phase III trials designed to evaluate the efficacy of a single intravitreal injection of GS010 (rAAV2/2-ND4) in subjects affected by LHON due to the G11778A mutation in the mitochondrial ND4 gene.
The primary endpoint will measure the difference in efficacy of GS010 in treated eyes compared to sham-treated eyes based on Best-Corrected Visual Acuity (BCVA), as measured with the ETDRS at 48 weeks post-injection. The patients’ LogMAR (Logarithm of the Minimal Angle of Resolution) scores, which are derived from the number of letters patients read on the ETDRS chart, will be used for statistical purposes. Both trials have been adequately powered to evaluate a clinically relevant difference of at least 15 ETDRS letters between treated and untreated eyes adjusted to baseline.
The secondary endpoints will involve the application of the primary analysis to best-seeing eyes that received GS010 compared to those receiving sham, and to worse-seeing eyes that received GS010 compared to those that received sham. Additionally, a categorical evaluation with a responder analysis will be evaluated, including the proportion of patients who maintain vision (< ETDRS 15L loss), the proportion of patients who gain 15 ETDRS letters from baseline and the proportion of patients with Snellen acuity of >20/200. Complementary vision metrics will include automated visual fields, optical coherence tomography, and color and contrast sensitivity, in addition to quality of life scales, bio-dissemination and the time course of immune response. By protocol, readouts for these endpoints are at 48, 72 and 96 weeks after injection.
The trials are conducted in parallel, in 37 subjects for REVERSE and 39 subjects for RESCUE, in 7 centers across the United States, the UK, France, Germany and Italy. Week 96 results are expected in 2019 for both trials, after which patients will be transferred to a long-term follow-up study that will last for three years.
ClinicalTrials.gov Identifiers:
REVERSE: NCT02652780
RESCUE: NCT02652767
About REFLECT
REFLECT is a multi-center, randomized, double-masked, placebo-controlled study to evaluate the safety and efficacy of bilateral injections of GS010 in subjects with LHON due to the NADH dehydrogenase 4 (ND4) mutation.
The trial is planned to enroll 90 patients with vision loss up to 1 year in duration and will be conducted in multiple centers in Europe and in the US.
In the active arm, GS010 will be administered as a single intravitreal injection to both eyes of each subject. In the placebo arm, GS010 will be administered as a single intravitreal injection to the first affected eye, while the fellow eye will receive a placebo injection.
The primary endpoint for the REFLECT trial is the BCVA reported in LogMAR at 1-Year post-treatment in the second-affected/not-yet-affected eye. The change from baseline in second-affected/not-yet-affected eyes receiving GS010 and placebo will be the primary response of interest. The secondary efficacy endpoints include: BCVA reported in LogMAR at 2-Years post-treatment in the second-affected/not-yet-affected eye compared to both placebo and the first-affected eye receiving GS010, OCT, color and contrast sensitivity and quality of life scales. The first subject was treated in March 2018.
ClinicalTrials.gov Identifiers:
REFLECT: NCT03293524
View source version on businesswire.com: https://www.businesswire.com/news/home/20190203005038/en/
Contacts
GenSight Biologics
Thomas Gidoin
Chief Financial Officer
tgidoin@gensight-biologics.com
+33 (0)1 76 21 72 20
RooneyPartners
Media Relations
Marion Janic
mjanic@rooneyco.com
+1-212-223-4017
Solebury Trout Group
US Investor Relations
Chad Rubin
crubin@troutgroup.com
+1-646-398-2947
James Palmer
Europe Investor Relations
j.palmer@orpheonfinance.com
+33 7 60 92 77 74
Source: GenSight Biologics








