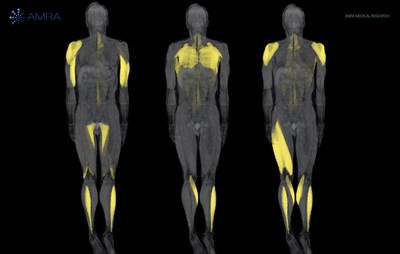
REACH, a Phase 3 Clinical Trial of losmapimod in Facioscapulohumeral Muscular Dystrophy (FSHD) utilizes AMRA’s Muscle Fat Infiltration (MFI) as a secondary endpoint.
|
REACH, a Phase 3 Clinical Trial of losmapimod in Facioscapulohumeral Muscular Dystrophy (FSHD) utilizes AMRA's Muscle Fat Infiltration (MFI) as a secondary endpoint. LINKÖPING, Sweden, July 13, 2022 /PRNewswire/ -- AMRA Medical, a digital health company delivering a new standard in body composition analysis through rapid whole-body magnetic resonance imaging (MRI), today announced that Fulcrum Therapeutics is using AMRA's muscle fat infiltration (MFI) as a secondary endpoint in the phase 3 clinical trial: REACH. The clinical trial is evaluating the efficacy and safety of losmapimod for the treatment of Facioscapulohumeral Muscular Dystrophy (FSHD). The results of the REACH trial will help move Fulcrum closer to delivering the first-ever medicine approved for FSHD and help establish whole-body MRI-based measurements as critical endpoints in clinical trials for neuromuscular diseases. FSHD is a progressive and debilitating neuromuscular disease for which there are no approved treatments. The disease is characterized by fat infiltration of skeletal muscle, impacting a patient's ability to perform daily tasks and live independently. Losmapimod is the first and only investigational medicine in clinical development, and with positive data, REACH could be the basis for approval. REACH is a randomized, double-blind, placebo-controlled, multi-national trial expected to enroll approximately 230 adults with FSHD. Patients will receive either losmapimod or placebo, and be evaluated over a 48-week treatment period. AMRA's MRI protocol will be used to produce a muscle fat infiltration (MFI) composite score for each patient as a secondary endpoint, with Reachable Workspace (RWS) as the primary endpoint. This is not the first time Fulcrum is leveraging AMRA's measurements. AMRA and Fulcrum have partnered with MRI in their losmapimod studies from early biomarker development, through ReDUX4 Phase 2b, and now to the REACH phase 3 trial. The ReDUX4 trial, which demonstrated that losmapimod slowed disease progression and improved function in people with FSHD, revealed RWS and MFI are reliable measures of disease progression. This led the EU and US regulatory agencies, including the FDA, to support the use of MFI composite scores in REACH. MFI composite scoring can help describe specific aspects of disease onset patterns, functional impairment, and disease progression between scans. The scores are generated following whole-body, whole-muscle MRI by combining muscle fat infiltration measurements from multiple muscles throughout the body. By converting all the MFI data for a given individual into a simplified score, researchers get meaningful information that is easier to interpret compared with trying to draw conclusions from 36 different MFI measurements for a given individual, let alone all of the 230 study participants. With AMRA, researchers can observe meaningful differences in both functional (RWS) and imaging (MFI) endpoints to gain more holistic insights and, ultimately, advance their clinical pipeline. The losmapimod journey demonstrates that progressive pharmaceutical companies can get valuable insights from AMRA's MRI-based body composition measurements when used in proof-of-concept studies through late-stage clinical trials. Learn how AMRA can help you advance metabolic and musculoskeletal clinical research and development by visiting AMRA’s website.
SOURCE AMRA Medical |