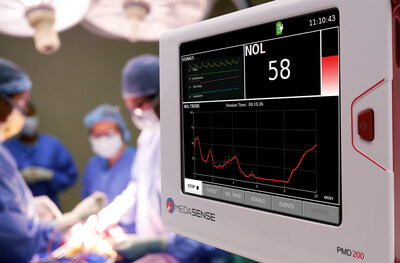
Medasense Biometrics Ltd. announced today that the U.S. Food and Drug Administration (FDA) has granted marketing authorization to the PMD-200 patient monitor.
|
Agency Clearance Paves Way for Commercialization in the United States of First and Only Authorized Device to Measure Pain in Anesthetized Patients Undergoing Surgery RAMAT GAN, Israel, Feb. 23, 2023 /PRNewswire/ -- Medasense Biometrics Ltd. announced today that the U.S. Food and Drug Administration (FDA) has granted marketing authorization to the PMD-200 patient monitor with NOL® technology developed for monitoring nociception (the physiological response to pain) through the De Novo premarket review pathway. NOL uses a unique multi-parametric sensor platform and advanced AI algorithms to convert complicated data into a patient's 'Signature of Pain.' The technology, which is currently utilized in operating rooms and high-acuity settings, where patients are under anesthesia and unable to communicate, enables clinicians to personalize pain management, control pain and avoid overmedication. NOL is the first and only market-authorized, adjunctive monitoring technology for the assessment of changes in nociception in adult patients receiving opioid or opioid sparing analgesics "NOL technology has the potential to improve the lives of hundreds of millions of patients worldwide, and we have already seen the impact it has had in multiple countries," said Galit Zuckerman-Stark, CEO & founder of Medasense. "Our mission is to help patients suffer less from pain and the adverse effects of pain medication. We are proud to offer the first and only measure of intraoperative pain (nociception) in the US, and to be able to offer meaningful innovation in the area of pain management to help patients undergoing surgery experience improved pain-related outcomes." Studies have shown that intraoperative NOL monitoring can reduce postoperative pain experienced by patients in the post anesthesia care unit,1,2 and potentially reduce costs of care.3 A recent publication showed that the odds of suffering severe post-operative pain are six times lower with NOL monitoring.4 This addresses a widespread need. It is estimated that 50% of surgical patients suffer from moderate to severe postoperative pain and 12% suffer adverse events due to pain relief medication.5 These can result in extended hospitalization, additional healthcare costs, and a 50% increase in hospital readmissions.6 "The anesthesia community has needed a technology like NOL for a long time," said Frank Overdyk, MSEE, MD, Charleston, SC. "We have devices that monitor depth of anesthesia, we have TOF cuffs to check for patient movement, but the missing piece of the puzzle is a way to monitor the effect of the opioid or opioid sparing analgesia. Relying on patient's heart rate and blood pressure is neither specific nor sensitive enough to pain. This technology as an adjunctive to clinical judgment will provide a window into the patient's nociceptive state during surgery so we can personalize the way we administer analgesia, improving the patient's recovery." The FDA market authorization was supported by pivotal clinical data demonstrating that NOL guided intraoperative analgesia as an adjunct to clinical judgment with clinical and vital signs resulted in improved postoperative pain scores in the post- anesthesia care unit in adult patients. About Medasense and NOL Technology Medasense Biometrics Ltd. (www.medasense.com) is a global technology company with its head office in Israel, that develops and produces breakthrough technology that enables clinicians to personalize pain management and avoid overmedication. Our mission is to improve patient outcomes, optimize pain management and relieve suffering in any care setting. Over 60,000 patients in operating rooms and intensive care have already been treated with Medasense's flagship product, the PMD-200™. The NOL® index is a unique platform that objectively monitors and quantifies the patient's pain response by means of a proprietary non-invasive sensor platform and artificial intelligence. Leveraging the NOL technology, the company has developed a pipeline of cloud-based solutions for clinics and homes addressing additional markets and collaboration opportunities for long-term follow-up of pain treatment. The PMD-200 is distributed in Western Europe by Medtronic, is cleared for marketing and distributed also in Canada, Latin America, Israel, UAE, and Turkey, and enables connectivity with Philips and Mindray patient monitors. Watch Medasense’s 1-minute video Dr.Frank Overdyk serves as a consultant Medical Director to Medasense. 1. Meijer, F.et (2020). Reduced postoperative pain using Nociception Level-guided fentanyl dosing during sevoflurane anaesthesia: a randomised controlled trial. British Journal of Anaesthesia, For further information please contact: Medasense Contact Media Contact: Photo - https://mma.prnewswire.com/media/2008564/Medasense_PMD_200_monitor.jpg
SOURCE Medasense Biometrics Ltd. |