GREENFIELD, Ind., Sept. 14, 2016 /PRNewswire/ -- Today, Elanco Animal Health, a division of Eli Lilly and Company (NYSE: LLY), reports on scientific and structural advancements made toward enhancing responsible antibiotic use and bringing new alternatives to decrease the need for shared-class antibiotics in livestock. In an update tracking progress of its 8-Point Antibiotic Stewardship Plan unveiled last year, Elanco announces the following actions:
- Completing label change submissions to remove growth promotion from shared-class antibiotics globally and to require veterinary oversight where infrastructure exists
- Created two new research teams and invested 90 percent of annual food animal research budget to deliver new alternatives
- Eight new candidates moving into Elanco's product development pipeline
- $2 million innovation incentive support against five livestock diseases
- Launched or expanded geographic availability of four antibiotic alternatives including vaccines, enzymes and a protein, and gained approval for two new animal-only antibiotics
- Creation of nutritional health business to protect animal health using the latest science to improve microbiome
"Elanco is committed to bringing greater clarity to issues around antibiotic stewardship and shaping science-based recommendations on responsible use, animal welfare, and the long term sustainability of the food system," said Jeff Simmons, president, Elanco Animal Health. "We have a responsibility to the health and welfare of animals, to treat the ones that get sick while safeguarding antibiotics for future generations through responsible use."
Bacteria resistance is an evolving science, and we must continue to better understand the drivers. Of the 18 organisms identified by the United States Centers for Disease Control and Prevention (CDC) as antibiotic resistance threats, just two of them were directly related to animal use of antibiotics1. Human, animal and environmental sectors all have a role to play in protecting antibiotics for the long-term. The Elanco 8-Point Antibiotic Stewardship Plan promotes responsible antibiotic use; decreasing the need for shared-class antibiotics; and bringing treatment alternatives to livestock producers to keep animals healthy while decreasing the need for shared-class antibiotics.
Among the scientific advances realized:
- Nearly 50 new development ideas have been brought forward for assessment, with 8 new candidates moving into Elanco's research and development pipeline and 21 additional candidates completing proof-of-concept studies over the next year.
- Research efforts focused on the greatest areas of unmet need for livestock producers where shared-class antibiotics are the only option today.
In 2016, Elanco also launched two animal-only antibiotics for intestinal diseases in pigs and poultry, as well as a completely new non-antibiotic alternative to reduce mastitis in dairy cows, the greatest use of shared-class antibiotics in the dairy industry today. These new products give livestock producers alternatives to protect animal health and welfare without impacting antibiotic treatment outcomes for people.
Elanco is also progressing on a number of other efforts to increase responsible use, including:
- Completed 75 label change submissions to remove growth promotion claims around the world. This includes full compliance with U.S. FDA Guidance 213 in advance of the end-of-year deadline.
- An additional 18 label change submissions will be completed in Latin America by end-of-year.
- Completed submission of 67 labels for shared class molecules to move products from over-the-counter to be under veterinary oversight in the United States, Canada and Brazil, the countries where over-the-counter use remains, and veterinary infrastructure exists.
New Commitments
"To continue progress made over the past year, Elanco is sustaining its commitment to finding alternatives to antibiotics," said Aaron Schacht, vice president of Elanco Research and Development. "Through our internal and external innovation efforts, we are poised to deliver a pipeline with a mix of preventatives and treatments that could help reduce the use of shared-class antibiotics."
25 New Viable Antibiotic Development Projects
By 2020, Elanco aims to deliver a total of 25 viable antibiotic alternative development projects that address critical unmet challenges in livestock production via alternatives to shared-class antibiotics. We believe we can provide solutions that address five of these disease challenges in a fundamentally new way by 2020.
Grand Challenge
Elanco will incentivize new innovation from many sources, including internal development, external partnerships and open source innovation.
The Elanco Advancing Antibiotics Alternatives Grand Challenge, in partnership with InnoCentive, will offer more than $2 million in support and incentives to find new innovation ideas for five of the most challenging livestock diseases that rely on shared-class antibiotics today, including:
- Liver abscesses in beef cattle
- Necrotic enteritis in poultry
- Coccidiosis in poultry
- Lawsonia in pigs
- Strep suis in pigs
The first challenges are now open for response and can be found here.
New Nutritional Health Business Unit
Finally, Elanco is creating a nutritional health business to protect and improve animal health using the latest science to improve gastrointestinal health and the microbiome. Early disease prevention with non-medicated feed additives, like enzymes, prebiotics and probiotics, can improve microbiome health, helping protect from disease even before clinical signs develop thus lessening the need for antibiotics. In addition to Elanco's current product in this space, the new business is anticipated to deliver two new non-antibiotic products to customers annually between 2017 and 2020.
Prevention 360: Aggregating Information, Interventions and Innovations
To help prevent diseases and aid early disease detection, Elanco is also introducing Prevention 360 to aggregate the many sources of production, health and post-harvest data. Prevention 360 will help more effectively identify early indicators of disease, develop alternative production practices and provide product solutions.
To learn more, join Elanco's informational webcast at 9 a.m. EDTSeptember 14 to participate in a question and answer session with Elanco experts, including Elanco President Jeff Simmons. Registration details are below:
Via Internet Webcast
http://event.on24.com/r.htm?e=1234689&s=1&k=6F1029C8F01C1FCDE9DF89701E8E33AB
Listen-Only Phone Numbers
Toll-Free: (844) 586-0543
Toll: (702) 425-5347
Conference ID: 58042765
About Elanco
Elanco provides comprehensive products and knowledge services to improve animal health and food-animal production in more than 70 countries around the world. We value innovation, both in scientific research and daily operations, and strive to cultivate a collaborative work environment for more than 6,500 employees worldwide. Together with our customers, we are committed to raising awareness about global food security, and celebrating and supporting the human-animal bond. Founded in 1954, Elanco is a division of Eli Lilly and Company. Our worldwide headquarters and research facilities are located in Greenfield, Indiana. Visit us at Elanco.com and EnoughMovement.com.
This press release contains forward-looking statements (as that term is defined in the Private Securities Litigation Reform Act of 1995) about the benefits of Elanco's antibiotic stewardship program and the potential of Elanco's product pipeline and research efforts for shared-class antibiotics, and reflects Elanco's and Lilly's current belief. However, as with any animal health product, there are substantial risks and uncertainties in the process of development and commercialization. Among other things, there can be no guarantee that future study results will be consistent with study findings to date, that the above referenced shared-class antibiotics will receive regulatory approvals or that the product pipeline will prove to be commercially successful. For further discussion of these and other risks and uncertainties, see Lilly's most recent Form 10-K and Form 10-Q filings with the United States Securities and Exchange Commission. Except as required by law, Lilly and Elanco undertake no duty to update forward-looking statements to reflect events after the date of this release.
1 CDC: http://www.cdc.gov/drugresistance/pdf/ar-threats-2013-508.pdf
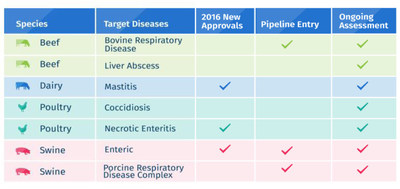

Photo - http://photos.prnewswire.com/prnh/20160913/407406-INFO
Logo - http://photos.prnewswire.com/prnh/20160913/407389LOGO
To view the original version on PR Newswire, visit:http://www.prnewswire.com/news-releases/elancos-antibiotic-stewardship-plan-yields-innovation-scientific-advancements-300327720.html
SOURCE Elanco Animal Health





