ST-PREX, SWITZERLAND--(Marketwire - April 06, 2009) - Edwards Lifesciences Corporation (NYSE: EW), a global leader in hemodynamic monitoring, today announced the availability of third generation software (G3) for its minimally invasive FloTrac cardiac monitoring system that enhances the product's accuracy when used in patients with sepsis and other critical illnesses. The new software draws upon state-of-the-art analysis and a broader patient database to enable the FloTrac system to automatically and accurately adjust to the radical changes that occur in these patients. Sepsis is a common, life-threatening condition that results from the body's response to infection, with more than 18 million cases of severe sepsis reported worldwide each year.
Results from the first multi-center validation study of the new FloTrac system software were recently presented at the 29th International Symposium on Intensive Care and Emergency Medicine (ISICEM) in Brussels, Belgium. "The software was shown to be accurate when used on patients with sepsis, even in the presence of very low peripheral vascular resistance," said Daniel De Backer, MD, Erasme University Hospital in Brussels, Belgium.
"Sepsis is the leading cause of death in the non-coronary intensive care unit and it is important to provide clinicians with an advanced tool that will help them treat this challenging patient population," said Carlyn D. Solomon, corporate vice president, critical care and vascular. "As a world leader in hemodynamic monitoring, we are constantly striving to enhance and evolve our technology to best meet the needs of critical care clinicians and their patients."
About the FloTrac System
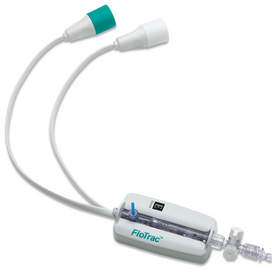
The FloTrac system is a global marketing leading minimally invasive monitoring system that provides continuous cardiac measurements of patients in a critical care environment. This easy-to-use system accesses patient data directly from an existing arterial line, a small catheter inserted into the patient's radial artery. Traditionally, this information is collected through a more invasive pulmonary artery catheter placed in the patient's heart. While clinicians continue to rely on Edwards' Swan-Ganz catheters as the gold standard for obtaining the most comprehensive level of cardiovascular monitoring, the FloTrac system is used to gather many of those same parameters earlier and less invasively -- enabling previously unmonitored patients to receive more advanced care.
About Edwards Lifesciences
Edwards Lifesciences is the global leader in the science of heart valves and hemodynamic monitoring, with more than five decades of experience in partnering with clinicians to develop life-saving innovations. Headquartered in Irvine, Calif., Edwards treats advanced cardiovascular disease with its market-leading heart valve therapies, and critical care and vascular technologies, which are sold in approximately 100 countries. The company's global brands include Carpentier-Edwards, Cosgrove-Edwards, Edwards SAPIEN, FloTrac, Fogarty, PERIMOUNT Magna and Swan-Ganz. Additional company information can be found at http://www.edwards.com.
Edwards is a trademark of Edwards Lifesciences Corporation. Edwards
Lifesciences, the stylized E logo, Carpentier-Edwards, Cosgrove-Edwards,
Edwards SAPIEN, FloTrac, Fogarty, PERIMOUNT Magna and Swan-Ganz are
trademarks of Edwards Lifesciences Corporation and are registered in the
United States Patent and Trademark Office.
Media Contact:
Amanda C. Fowler
949-250-5070
Investor Contact:
David K. Erickson
949-250-6826