Talk entitled “Safety and Response of an Evans Blue-modified 177Lu-labeled Octreotate (EBTATE) in Treatment of Metastatic Neuroendocrine Tumors: A Pilot Prospective Study” at the Society of Nuclear Medicine and Molecular Imaging (SNMMI) in Anaheim this June*.

WEST CHESTER, Pa.--(BUSINESS WIRE)-- Molecular Targeting Technologies, Inc. (MTTI) announced today that Professor Zhaohui Zhu et. al. of the Department of Nuclear Medicine, Peking Union Medical College Hospital (PUMC), Beijing, China will present a talk entitled “Safety and Response of an Evans Blue-modified 177Lu-labeled Octreotate (EBTATE) in Treatment of Metastatic Neuroendocrine Tumors: A Pilot Prospective Study” at the Society of Nuclear Medicine and Molecular Imaging (SNMMI) in Anaheim this June*.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20190624005007/en/
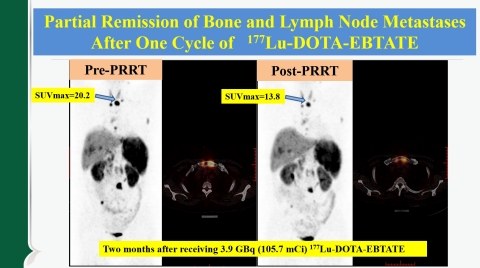
Partial Remission of Bone and Lymph Node Metastases After One Cycle of EBTATE (PRRT-peptide receptor radionuclide therapy). (Graphic: Business Wire)
MTTI received an exclusive worldwide commercialization license from NIH for this technology. This patent estate covers our EBTATE radiotherapeutic. Neuroendocrine neoplasm (NEN) treatment is among its potential uses.
The talk describes results of a dose escalation study comparing MTTI’s EBTATE to Lutathera®. EBTATE was designed to have prolonged circulation half-life and increased neuroendocrine tumor accumulation versus its predecessor. Dr. Zhu’s team studied 26 patients in three groups with metastatic neuroendocrine neoplasms (NENs), comparing two dose levels of EBTATE to Lutathera® against Common Toxicity Criteria (CTC) for tolerability.
Qingxing Liu and Zhaohui Zhu highlighted, “One treatment cycle of 1.85 GBq (50 mCi) or 3.70 GBq (100 mCi) of EBTATE seems to be well tolerated and more effective than 3.70 GBq (100 mCi) of Lutathera®.”
“Based on this early work, EBTATE could be the next, more effective, innovation in radiolabeled molecules for NEN. We’re excited about this breakthrough and thank PUMC’s colleagues for their exceptional contribution in our concerted effort to develop this robust therapy,” said Chris Pak, President & CEO of MTTI.
MTTI is a privately held biotechnology company focused on the acquisition and development of novel technologies for treatment and diagnosis of human diseases. More information: www.mtarget.com.
*Oral presentation by Qingxin Liu, Zhaohui Zhu and Xiaoyuan Chen et. al.
|
Response evaluation Group |
||||||||||||
|
|
Response |
|||||||||||
|
CR |
PR |
SD |
PD | |||||||||
| 100mCi TATE | 0 | 16.7% (1/6) | 50% (3/6) | 33.3% (2/6) | ||||||||
| 50mCi EBTATE | 0 | 50% (3/6) | 50% (3/6) | 0 | ||||||||
| 100mCi EBTATE | 0 | 50% (7/14) | 42.9% (6/14) | 7.1% (1/14) | ||||||||
TATE: Lutathera®; EBTATE: MTTI’s EBTATE;
CR: Complete response; PR: partial response; SD: stable disease; PD: progressive disease
View source version on businesswire.com: https://www.businesswire.com/news/home/20190624005007/en/
Source: Molecular Targeting Technologies, Inc.






