Drug Development
Sanofi will take venglustat to regulators for Gaucher disease but an application for Fabry disease is less clear after the failure of a Phase III trial.
FEATURED STORIES
Together with robust data-driven modeling, rethinking regulation and data use could push forward a notoriously challenging field.
From opening new therapeutic mechanisms to repairing neuronal damage, investigational molecules from Ventyx Therapeutics, AC Immune, Gain Therapeutics and more could shape the future of Parkinson’s disease treatment.
As drug candidates discovered via AI move into later-stage clinical trials, the technology seems to be doing as promised: speeding drug development.
Subscribe to ClinicaSpace
Clinical trial results, research news, the latest in cancer and cell and gene therapy, in your inbox every Monday
THE LATEST
This week’s announcement includes a step to the side for CMO Amit Rakhit, who will transition to the biotech’s advisory board.
The saga continues for Biogen’s Alzheimer’s drug Aduhelm as the U.S. Food and Drug Administration approved an updated label that emphasizes the disease stages for which the drug was approved.
The study, which compared the same treatment combination to pomalidomide plus dexamethasone covered 495 patients from over a hundred hospitals in 21 countries.
A Phase II study shows the vaccine by Imugene Limited improved survival rates and overall response in patients with HER2-positive stomach or gastroesophageal junction cancer.
Sinovac’s CoronaVac was the first COVID-19 vaccine approved in China for emergency use back in June 2020.
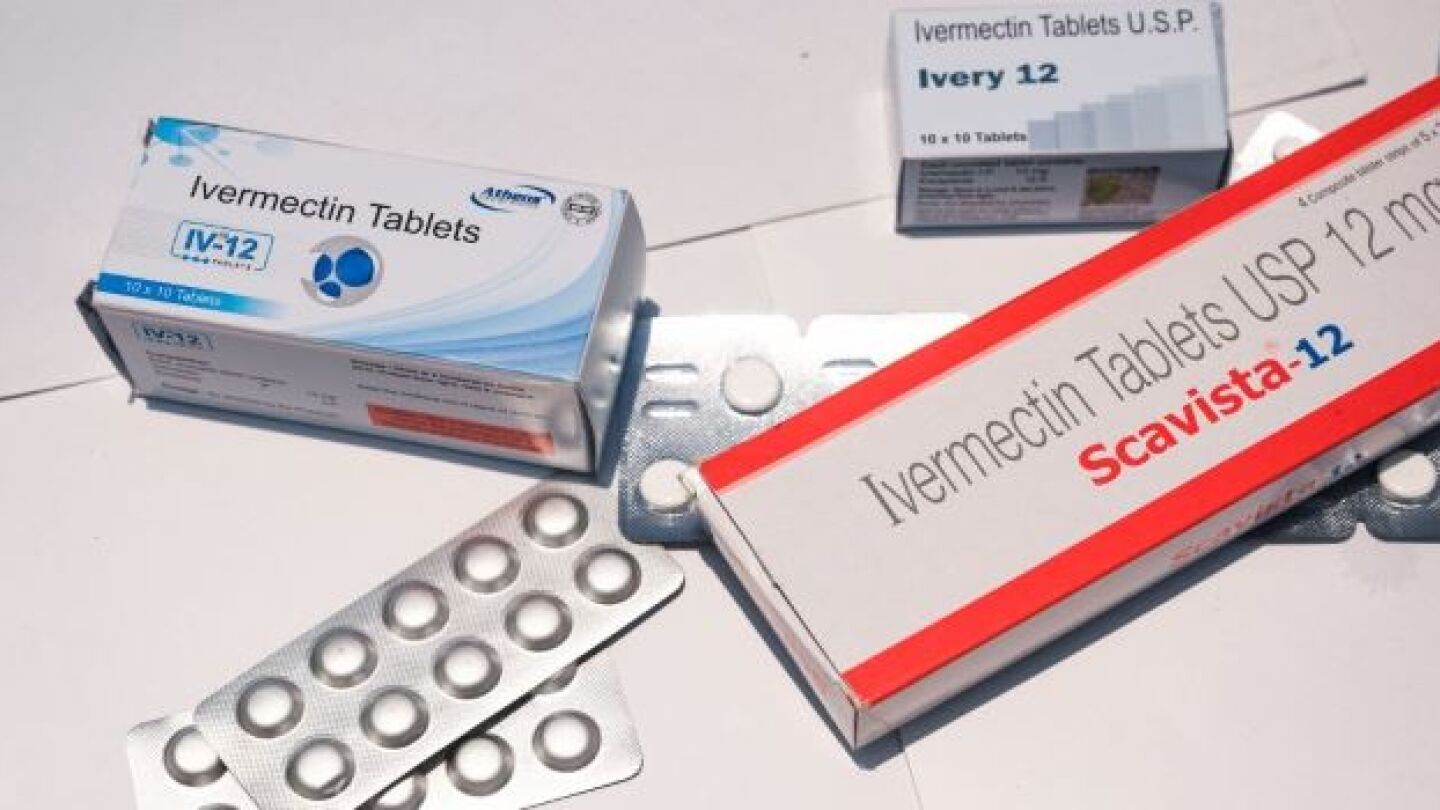
Ivermectin has become a contentious issue as to whether it effectively prevents and treats COVID-19. Several studies suggest it is effective, while others have indicated it is not.
The vaccine demonstrated efficacy in mild, moderate, and severe COVID-19 of 77.8% with efficacy against severe disease of 93.4%.
University of Oxford researchers have begun clinical trials for a new vaccine that can potentially target a wide range of HIV variants.
The WHO is calling for manufacturers to cut prices and increase the availability of supplies to low- and middle-income nations to parts of the world where COVID-19 continues to surge.
The expanded approval was made based on positive data from a second interim analysis of the Phase II KEYNOTE-629 trial.