Drug Development
Infigratinib topped “even the most optimistic expectations” for efficacy and safety in the late-stage PROPEL 3 study in achondroplasia, Truist Securities analysts said Thursday.
FEATURED STORIES
Analysts, investors and scientists are eager for Biogen’s 2026 BIIB080 readout. Even if successful, executives warn that there are many more steps before the Alzheimer’s therapy could reach the market.
With a clutch of key data and planned regulatory applications this year from Avidity Biosciences, REGENXBIO and Capricor Therapeutics, CureDuchenne CSO Michael Kelly sees “momentum” in the Duchenne muscular dystrophy pipeline, as Sarepta’s Elevidys leaves the door open.
After advancing in lockstep through the pandemic, the fortunes of the biotechs have diverged as their use of COVID-19 windfalls has taken shape.
Subscribe to ClinicaSpace
Clinical trial results, research news, the latest in cancer and cell and gene therapy, in your inbox every Monday
THE LATEST
BridgeBio Pharma, Inc. (Nasdaq: BBIO) (BridgeBio), today announced positive interim results from PROPEL 2, a Phase 2 trial of the investigational therapy infigratinib in children with achondroplasia.
Researchers at the Institut Pasteur in Paris, France have found that the COVID-19 virus enters the brain through nanotubes to cause debilitating neurologic symptoms.
Here’s a look at the latest Long COVID research and the reemergence of a therapy whose efficacy has yet to be proven.
Ionis announced that the FDA has accepted its NDA for tofersen for SOD1-ALS. The application has been given the priority review designation and an action date of January 25, 2023.
BridgeBio announces positive Phase II trial results for achondroplasia, Astellas-Seagen announces promising outcomes from Padcev Plus Keytruda Trial and Kelun-Biotech’s exclusive MSD deal.
The company is readjusting its strategy as it seeks ways to preserve share value. The goal to preserve as much as the $60 million it has in cash and cash equivalents as of the year’s second quarter.
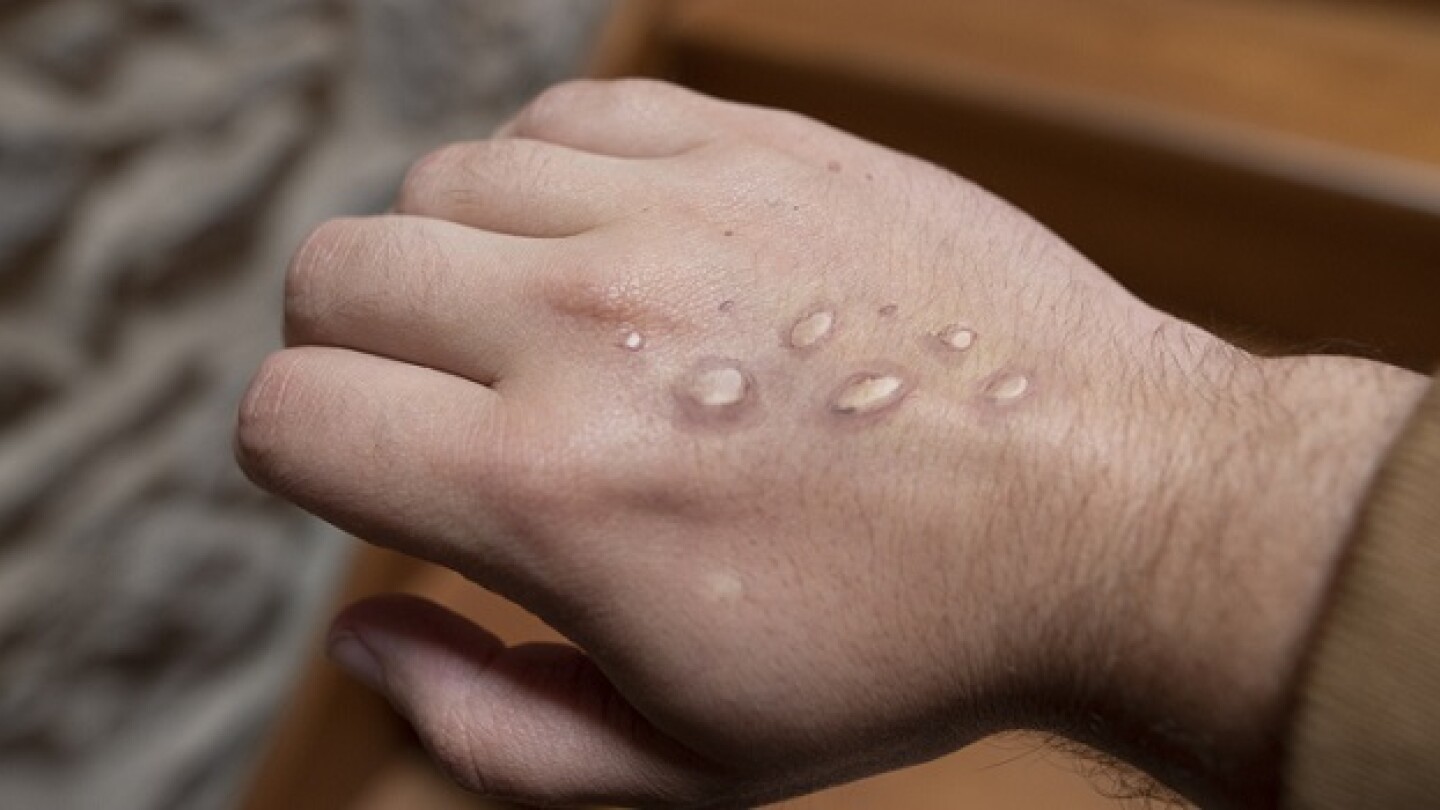
WHO declared the monkeypox outbreak a “public health emergency of international concern [PHEIC].” As COVID-19 wanes, Tonix, SIGA, Emergent Bio and others are now targeting monkeypox.
Here’s a look at global biopharma company news stories including Rona Therapeutics, Genuine Bio and NEOsphere Bio.
Daiichi-Sankyo and AstraZeneca’s sBLA for HER2 directed Enhertu accepted by FDA. Novartis gets BLA acceptance for MS biosimilar. HOOKIPA’s IND for prostate cancer immunotherapy candidate accepted
A study published this month in Science discusses data that may support a devious alter ego of T-cells present within colorectal cancers that appear to inhibit and promote tumor progression.