Trethera Corporation (“Trethera”) received a unanimous recommendation from the independent safety review committee (“SRC”) to continue clinical trial advancement of Trethera’s lead development asset, TRE-515, its first-in-class deoxycytidine kinase (“dCK”) inhibitor.
LOS ANGELES, March 15, 2023 (GLOBE NEWSWIRE) -- Trethera Corporation (“Trethera”), a clinical stage biopharmaceutical company committed to developing novel drugs targeting nucleotide metabolism for the treatment of cancer and autoimmune diseases, received a unanimous recommendation from the independent safety review committee (“SRC”) to continue clinical trial advancement of Trethera’s lead development asset, TRE-515, its first-in-class deoxycytidine kinase (“dCK”) inhibitor. This recommendation follows favorable results from the company’s Phase 1a dose escalation clinical trial in high risk, heavily pretreated, patients with solid tumor malignancies.
The Phase 1a open-label trial enrolled 19 patients with various solid tumors in the planned five dose escalation cohorts. The study’s primary endpoints were to determine the safety and maximum tolerability of TRE-515 when administered orally once daily as a single agent. The secondary endpoints were to (i) establish a recommended Phase 2 dose, (ii) characterize the pharmacokinetics/pharmacodynamics, and (iii) evaluate preliminary antitumor activity. The protocol also included exploratory objectives such as measuring biomarkers of target engagement.
In the trial, TRE-515 has demonstrated a manageable safety and tolerability profile resulting in an eight-fold increase above the initial starting dose. While multiple patients remain on therapy today, details of the most recent SRC and clinical data include:
Safety: Patients (N=19) tolerated TRE-515 well, with adverse events being limited, transient and manageable. No dose-limiting clinical or laboratory toxicities were observed in any treated patient who received daily doses of 40 mg up to 320 mg. Two patients were dosed continuously for over 200 days while another two for over 100 days with acceptable safety profiles.
Dose: TRE-515 had nearly dose proportional exposure over the 40 mg to 320 mg dose range, displaying favorable pharmacokinetics characterized by rapid absorption (Tmax less than 2 hours) and a plasma half-life of over 6 hours supporting once daily oral administration. Exposure levels were favorable relative to potency, even at the lowest dose of 40 mg.
Antitumor Activity: A secondary endpoint of the study included signaling for biological effect. Over all dose levels studied, there were five (N=5) cases of stable disease per RECIST v1.1 criteria reported. The majority of these patients had been heavily pretreated, meaning 5th line therapy or later, with extensive metastases.
Biomarkers: Plasma nucleoside concentrations, the substrate for the enzyme dCK, provided compelling evidence (p value <0.0001) of target engagement. Nucleosides potentially represent a readily accessible liquid biomarker for monitoring response during therapy, adjusting dose levels, and detecting a loss of response.
Overall, these data demonstrate that TRE-515 was well tolerated and showed signs of activity in heavily pretreated patients with high tumor burdens. Further, TRE-515 had predictable exposure and proven evidence of on-target activity. Taken together, the data support further evaluation of TRE-515. Given the promising data, as well as the SRC unanimous recommendation, discussions with the Food and Drug Agency (“FDA”) have initiated to (i) continue dose escalation, (ii) increase the number of patients, and (iii) include new clinical sites, thereby providing the opportunity for additional patients to receive treatment. After additional dose escalation concludes, the trial will enter dose expansion and dose range finding to further establish the Recommended Phase 2 Dose (RP2D).
“Based on the first-in-class mechanism of action, we believe TRE-515 has breakthrough potential. We’ve seen strong trial enrollment and remain optimistic for how TRE-515 can impact the future of cancer care,” said Dr. Zev Wainberg, principal investigator of the trial and medical oncologist at the University of California Los Angeles (UCLA). “We are especially excited that this data set included advanced biomarkers that will enable us to potentially measure drug impact and malignant progression.”
Dr. Tim Donahue, Trethera Board member and UCLA Chief of Surgical Oncology, commented, “These results establish the initial positive data set for safety and tolerability, suggesting that TRE-515 will have a wide therapeutic window, something uncommon for a cancer therapeutic. Moreover, the observations of first antitumor activity, such as those in a high-risk aggressive pancreatic cancer as 6th line therapy, indicate the therapeutic potential of TRE-515 for patients with considerable cancer burden.”
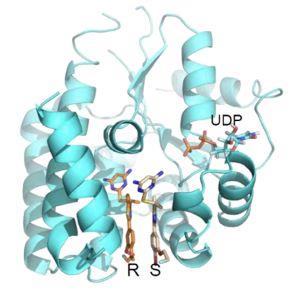
Figure 1. Co-crystal 3D structure of the drug bound to the target enzyme, dCK, at the deoxycytidine binding site.
TRE-515 is an orally delivered therapeutic engineered to inhibit dCK, the key enzyme in the nucleoside salvage pathway. A common characteristic of tumor cells in solid malignancies and pathological immune cells in autoimmune diseases is the requirement for elevated nucleotide levels to support abnormal and accelerated cell division. In contrast, dCK activity is not required in most healthy adult human cells. Mediated by the rate limiting enzyme, dCK, the nucleoside salvage pathway may play a pivotal role in limiting the rapid cell proliferation of cancer cells and aberrant activated lymphocytes, suggesting dCK as a potential therapeutic target with expected enhanced safety.
“We are pleased to successfully complete the dose escalation period, and excited to continue the study in difficult to treat cancers, as it moves us closer to key FDA submissions which are important steps in bringing this novel, first-in-pathway therapy closer to patients,” added Dr. Ken Schultz, Trethera Chairman and CEO. “Simultaneous with completing the current study, Trethera is optimizing the biomarker assays and manufacturing processes in preparation for Phase 2 proof-of-concept trials.”
Trethera looks forward to presenting the final data package at a future oncology conference.
About Trethera
Trethera is a clinical stage privately held biopharmaceutical company dedicated to pioneering the development of novel treatments for autoimmune diseases and cancers. Founded by prominent UCLA scientists, Trethera is led by experienced management and board members. Trethera's innovative approach to targeting nucleotide metabolism led to the development of TRE-515, an orally administered capsule twice designated by the FDA as an Orphan Drug. TRE-515 is a first-in-class clinical stage drug that inhibits deoxycytidine kinase (dCK), the rate-limiting enzyme in the nucleoside salvage pathway, one of two biosynthetic pathways that generate DNA precursors. It is believed that some forms of cancer may be preferentially dependent on the salvage pathway to support tumor growth, and certain autoimmune diseases, such as multiple sclerosis, might also respond to TRE-515 treatment. Trethera is developing TRE-515 for use as a monotherapy or in combination, to precisely target a metabolic vulnerability of cancer or autoimmune diseases that will transform outcomes for patients.
For more information, please visit us at trethera.com or e-mail Investor Relations at ir@trethera.com.
Note on Forward-Looking Statements
All statements other than statements of historical facts included in this press release that address activities, events or developments that Trethera believes or anticipates will or may occur in the future are “forward-looking statements,” which may often, but not always, be identified by the use of such words as "may," "might," "will," "will likely result," "would," "should," "estimate," "plan," "project," "forecast," "intend," "expect," "anticipate," "believe," "seek," "continue," "target" or the negative of such terms or other similar expressions. Although Trethera has a reasonable basis for the forward-looking statements contained herein, Trethera cautions that such statements are based on current expectations about future events and are subject to risks, uncertainties and factors relating to medical and scientific research, all of which are difficult to predict and many of which are beyond Trethera’s control, that may cause actual results to differ materially from those expressed or implied by the forward-looking statements in this press release. These potential risks and uncertainties include, without limitation: the extent to which development of any novel cancer therapies or therapies for autoimmune diseases succeeds; whether Trethera would obtain the necessary regulatory approvals to commence human trials or commercialize TRE-515 or any novel therapies resulting from such research; Trethera successfully implementing its growth strategy, including that relating to its disease therapies; the effects of the global Covid-19 pandemic; changes in economic conditions; competition; and risks and uncertainties applicable to the business of Trethera. The statements in this press release speak only as of the date hereof and Trethera does not undertake any obligation to update, amend or clarify these forward-looking statements whether as a result of new information, future events or otherwise. The Company intends that all forward-looking statements be subject to the safe-harbor provisions of the Private Securities Litigation Reform Act of 1995.
A photo accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/68a7f685-09c5-466d-91d5-de12692074aa