The brain cancer, glioblastoma, is a fierce and formidable opponent. Its millions of victims include Senator John McCain, President Biden’s son, Beau, and famed film critic Gene Siskel, to name just a few.
COLD SPRING HARBOR, N.Y., Dec. 21, 2022 /PRNewswire/ -- The brain cancer, glioblastoma, is a fierce and formidable opponent. Its millions of victims include Senator John McCain, President Biden's son, Beau, and famed film critic Gene Siskel, to name just a few. Most patients succumb within two years and few make it past five, a statistic that hasn't improved in decades due to lack of effective treatment options.
"The aggressiveness of glioblastoma is notorious," says Cold Spring Harbor Laboratory (CSHL) Professor Alea Mills. "The norm is to do surgery, treat with harsh drugs, and just hope for the best." But now, Mills and her colleagues have discovered in this deadly cancer a vulnerability, known as BRD8, that may finally lead to new treatment options and better patient outcomes.
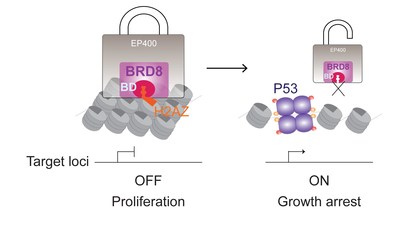
The CSHL team recently solved a decades-old mystery surrounding glioblastoma's aggressiveness by linking the BRD8 protein to another protein, named P53. A staple in the body's natural cancer defenses, P53 prevents cells from overgrowing and turning into tumors. Almost all cancers depend on P53 becoming mutated and thus disabled. But weirdly, in the majority of glioblastoma cases, P53 is unscathed. "So why does this cancer act like P53 is broken?" asked CSHL postdoctoral fellow Xueqin Sun. This critical question led Mills' team to discover that BRD8 had gone rogue in glioblastoma, crippling P53 in a completely new way.
BRD8 shuts down access to genes in chromosomes. If a gene is wound up tightly, it cannot be used—it's as if it were "asleep." Mills and her team revealed that BRD8 was inappropriately active in glioblastoma, keeping many of P53's critical anticancer defenses at rest. When the researchers inactivated BRD8 via genome editing, P53's "arsenal" suddenly woke up and began blocking tumor growth.
"It's like BRD8 is saying 'NO ENTRY' to P53's tumor-preventing power, but when we hit BRD8 in the right way—go in there almost like a scalpel, but molecularly—the tumor is annihilated," Mills explains. She and her team implanted tumor cells from glioblastoma patients into mice and watched the tumors grow in the brain. When BRD8 was inactivated, P53 was unlocked—the tumors stopped growing and the mice lived longer.
The finding suggests that drugs targeting the heart of BRD8 could work against glioblastoma. Mills hopes her team's discovery will help turn this deadly brain cancer into a treatable disease and for the first time in a generation, extend the life expectancy of patients who are diagnosed with it.
Founded in 1890, Cold Spring Harbor Laboratory has shaped contemporary biomedical research and education with programs in cancer, neuroscience, plant biology and quantitative biology. Home to eight Nobel Prize winners, the private, not-for-profit Laboratory employs 1,000 people including 600 scientists, students and technicians. For more information, visit www.cshl.edu
![]() View original content to download multimedia:https://www.prnewswire.com/news-releases/cracking-the-mystery-behind-a-deadly-brain-cancer-301705831.html
View original content to download multimedia:https://www.prnewswire.com/news-releases/cracking-the-mystery-behind-a-deadly-brain-cancer-301705831.html
SOURCE Cold Spring Harbor Laboratory