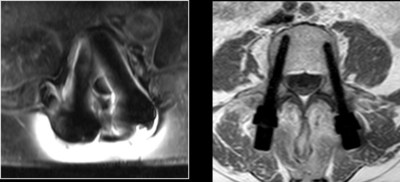
CarboFix has announced today that the U.S. Food and Drug Administration (FDA) has cleared its CarboClear® Carbon Fiber Fenestrated Pedicle Screw System used in the treatment of patients with advanced stage spinal tumors.
|
DOVER, Delaware, Sept. 24, 2019 /PRNewswire/ -- CarboFix has announced today that the U.S. Food and Drug Administration (FDA) has cleared its CarboClear® Carbon Fiber Fenestrated Pedicle Screw System used in the treatment of patients with advanced stage spinal tumors.
CarboClear® Fenestrated Pedicle Screws are used in conjunction with a high viscosity, radiopaque, PMMA bone cement (Teknimed's High V+ Cement), which is also sold by CarboFix. Pedicle Screw fixation in patients with advanced stage tumors may be challenging due to compromised bone. The use of PMMA-augmented CarboClear Fenestrated Screws provides for improved fixation. The CarboClear® System is the only pedicle screw system fully made of carbon fibers. Carbon fiber implants are proposing unique advantages to the oncological patients and their physicians. Among those advantages are: enhanced radiation therapy planning abilities, allowing radiation treatment to optimize the radiation dose to the tumor with minimal collateral tissue damage, and enhanced follow up abilities due to artifacts-free imaging. In addition, the implants provide unparalleled fatigue strength to support the impaired healing process in those patients, as well as compatibility with particle radiation (proton and carbon ion) and other stereotactic radio-surgery modalities. Indications for Use: When used in conjunction with High V+ Bone Cement, the CarboClear Fenestrated Pedicle Screws are intended to restore the integrity of the spinal column even in the absence of fusion for a limited time period in patients with advanced stage tumors involving the thoracic and lumbar spine in whom life expectancy is of insufficient duration to permit achievement of fusion. CarboClear Fenestrated Pedicle Screws augmented with High V+ Bone Cement are for use at spinal levels where the structural integrity of the spine is not severely compromised. To learn more about the CarboClear® System and the new CarboClear® Fenestrated Pedicle System, stop by booth #4805 during the NASS 2019 in Chicago. About CarboFix CarboFix is the world leading company in developing, manufacturing and marketing innovative carbon fiber spine and long bones solutions. The company products are cleared by the FDA and other regulatory authorities, and are being sold worldwide. For more information, please visit www.carbo-fix.com, or contact: Richard Doucette
SOURCE CarboFix |