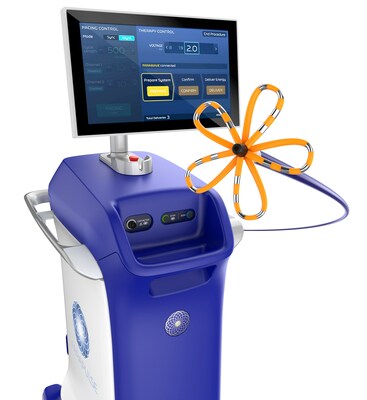
Boston Scientific Corporation (NYSE: BSX) announced it has received U.S. Food and Drug Administration (FDA) approval for the FARAPULSE™ Pulsed Field Ablation (PFA) System.
|
|
| [31-January-2024] |
|
Strong clinical evidence base, largest volume of real-world use reinforce safety, efficacy and efficiency advantages of the system MARLBOROUGH, Mass., Jan. 31, 2024 /PRNewswire/ -- Boston Scientific Corporation (NYSE: BSX) announced it has received U.S. Food and Drug Administration (FDA) approval for the FARAPULSE™ Pulsed Field Ablation (PFA) System. The FARAPULSE PFA System is indicated for the isolation of pulmonary veins in the treatment of drug-refractory, recurrent, symptomatic, paroxysmal (i.e., intermittent) atrial fibrillation (AF) and is a unique new alternative to standard-of-care thermal ablation treatment. "The approval of the FARAPULSE PFA System marks an important milestone for the millions of people living with paroxysmal AF and is an incredible opportunity to bring the first PFA system designed and built solely for this type of ablation therapy to physicians in the U.S.," said Nick Spadea-Anello, president, Electrophysiology, Boston Scientific. "A high bar has been set by performance of the system in clinical and commercial settings – where more than 40,000 patients have been treated to date – and we look forward to continuing to lead the way with this differentiated technology in the growing PFA space." During a traditional ablation procedure, a catheter is guided to the interior of the heart and generates extreme temperatures – hot or cold – to destroy targeted areas associated with abnormal heart rhythms. The FARAPULSE PFA System, however, relies on tissue-selective, non-thermal electric fields to ablate heart tissue and avoid damage to surrounding structures. Positive 12-month data from the pivotal ADVENT clinical trial – the first randomized clinical trial to directly compare the efficacy and safety of the system against standard-of-care ablation – found that therapy with the device was as safe and effective as conventional thermal ablation, with statistically shorter ablation times and a quick learning curve for physicians. Additional real-world data from more than 17,000 patients in the MANIFEST-17K registry demonstrated continued real-world safety of the system, with no reports of permanent phrenic nerve palsy, pulmonary vein stenosis or esophageal injury. "Within the ADVENT clinical trial, the FARAPULSE PFA System was shown to be a safe, effective and efficient option for treating paroxysmal AF, and extensive global real-world use has mirrored that profile," said Vivek Reddy, M.D., director of electrophysiology, Mount Sinai Fuster Heart Hospital, New York. "Tissue preferentiality and long-term efficacy, combined with markedly shorter procedure times and learning curves, position the FARAPULSE PFA System with strong potential to become a practice-changing technology for both U.S. physicians and patients alike." The FARAPULSE PFA System delivers pulsed field energy and consists of the FARAWAVE™ Ablation Catheter, the FARASTAR™ Ablation Generator, and the FARADRIVE™ Steerable Sheath, which is complemented by the VersaCross Connect™ Access Solution for the FARADRIVE Steerable Sheath to provide safe and efficient access to the left side of the heart during procedures with the system. The FARAWAVE catheter is used to treat a range of pulmonary vein anatomies using an over-the-wire catheter with variable basket and flower shapes, allowing the device to adapt to individual patient anatomies. These configurations reinforce ease-of-use for physicians and promote reproducible procedures between operators. Boston Scientific completed enrollment in the first phase of the ADVANTAGE AF clinical trial in the third quarter of 2023, which is studying the system for the treatment of patients with drug-refractory, symptomatic, persistent AF, and commenced enrollment in a second phase of the study to evaluate the safety and effectiveness of adjunctive use of the FARAPOINT™ PFA Catheter for cavotricuspid isthmus (CTI) ablations, a procedure used to treat atrial flutter. The company also recently commenced the AVANT GUARD clinical trial to evaluate the safety and efficacy of the system as a first-line treatment for persistent AF compared to anti-arrhythmic drug therapy. The FARAPULSE PFA System was granted Breakthrough Device Designation from the Center for Devices and Radiological Health (CDRH) of the U.S. FDA in 2019 and received CE Mark in 2021. Boston Scientific plans to immediately launch the system in the U.S. The company is developing a navigation-enabled version of the FARAWAVE catheter alongside the FARAVIEW™ Software Module and anticipates regulatory approval in 2024. More information on the FARAPULSE PFA System is available here. About Boston Scientific Cautionary Statement Regarding Forward-Looking Statements This press release contains forward-looking statements within the meaning of Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934. Forward-looking statements may be identified by words like "anticipate," "expect," "project," "believe," "plan," "estimate," "intend" and similar words. These forward-looking statements are based on our beliefs, assumptions and estimates using information available to us at the time and are not intended to be guarantees of future events or performance. These forward-looking statements include, among other things, statements regarding our business plans and product performance and impact, new and anticipated product approvals and launches, and clinical trials. If our underlying assumptions turn out to be incorrect, or if certain risks or uncertainties materialize, actual results could vary materially from the expectations and projections expressed or implied by our forward-looking statements. These factors, in some cases, have affected and in the future (together with other factors) could affect our ability to implement our business strategy and may cause actual results to differ materially from those contemplated by the statements expressed in this press release. As a result, readers are cautioned not to place undue reliance on any of our forward-looking statements. Factors that may cause such differences include, among other things: future economic, competitive, reimbursement and regulatory conditions; manufacturing, distribution and supply chain disruptions and cost increases; new product introductions; expected procedural volumes; demographic trends; intellectual property; litigation; financial market conditions; and future business decisions made by us and our competitors. All of these factors are difficult or impossible to predict accurately and many of them are beyond our control. For a further list and description of these and other important risks and uncertainties that may affect our future operations, see Part I, Item 1A – Risk Factors in our most recent Annual Report on Form 10-K filed with the Securities and Exchange Commission, which we may update in Part II, Item 1A – Risk Factors in Quarterly Reports on Form 10-Q we have filed or will file hereafter. We disclaim any intention or obligation to publicly update or revise any forward-looking statements to reflect any change in our expectations or in events, conditions or circumstances on which those expectations may be based, or that may affect the likelihood that actual results will differ from those contained in the forward-looking statements. This cautionary statement is applicable to all forward-looking statements contained in this document. CONTACTS: Lauren Tengler *Dr. Vivek Reddy is a paid consultant of Boston Scientific Corporation. He has not been compensated in connection with this press release.
SOURCE Boston Scientific Corporation |
||
Company Codes: NYSE:BSX |