BERLIN--(Marketwire - January 22, 2013) - BIOTRONIK, a leading manufacturer of innovative medical technology, today announced the start of the large-scale international MATRIX study (Management and Detection of Atrial TachyarRhythmias in Patients Implanted with BIOTRONIK DX Systems).
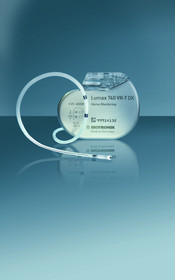
The study will collect data from patients implanted with BIOTRONIK's Lumax 740 or 540 VR-T DX implantable cardioverter-defibrillator (ICD) devices, which feature complete atrial sensing and detection in a single-lead system. MATRIX is a prospective open study that will include 2,000 patients in 100 sites throughout Europe, Latin America, Canada, Israel and Japan for up to four years. Patients will be followed for two years after enrolment in an unbiased, real-life setting while device data and clinical events are being continuously recorded.
Due to their asymptomatic and paroxysmal nature, atrial tachycardias (AT) are often overlooked in their early stages. The long-term observational MATRIX study will explore the enhanced features and unique capabilities of the Lumax DX ICD system from BIOTRONIK, focusing on detection and management of atrial fibrillation (AF) for optimized patient management and reduction of inappropriate shocks. In addition, diagnostic information continuously collected from the atrium will provide useful insight into the development and subsequent management of atrial fibrillation and its associated complications.
"The DX feature in BIOTRONIK's ICDs promises a significant breakthrough for patients," said Prof. Dr. Gerhard Hindricks, University of Leipzig--Heart Center, Department of Rhythmology, Germany. "It not only lowers a patient's AF-associated risks, but also avoids the risks associated with implantation of a second lead. Plus, you gain a full range of state-of-the-art algorithms to avoid inappropriate therapies."
Undetected and asymptomatic AF bears an increased risk of severe complications such as stroke or the risk of inappropriate device therapies. Since standard single-chamber ICDs use only ventricular information and ignore atrial events to make therapy decisions, the device may misclassify these as ventricular tachycardias and deliver inappropriate shocks. Lumax DX can discriminate between supraventricular tachycardias (SVTs), atrial fibrillation and atrial flutter with its SMART Detection® algorithm, helping to safely reduce this risk.
Due to the still significant risk of additional complications associated with the atrial lead, implantation of a dual-chamber ICD is restricted by current guidelines for indications that require additional pacing capabilities. This could potentially lead to a number of classic and secondary prevention indications being excluded from receiving the additional diagnostics that the Lumax DX offers.
"BIOTRONIK's new Lumax DX ICD system fills an important gap, providing diagnostic information from the atrium in a single-lead system, as well as enhanced care via BIOTRONIK Home Monitoring® -- a system that remotely transmits patient and device status data automatically on a daily basis," explained Hindricks.
"With our DX technology, we have once again proven our drive for innovation, clinical excellence, and ability to shape the future of medical device therapy," said Christoph Böhmer, President International, BIOTRONIK. "The Lumax DX offers an outstanding, innovative, unsurpassed engineering design that is saving thousands of patients' lives around the world. By utilizing data available from the device's home monitoring downloads, the MATRIX study will not only provide empirical data to support the efficacy and safety of the single-chamber Lumax DX system, but will also contribute to open questions in current AF research and thus promote scientific progress in this important area."
About BIOTRONIK SE & Co. KG
As one of the world's leading manufacturers of cardiovascular medical devices, BIOTRONIK is headquartered in Berlin, Germany, and represented in over 100 countries by its global workforce of more than 5600 employees. Several million heart patients around the world have received BIOTRONIK implants, designed to save and improve the quality of their lives. Since its development of the first German pacemaker in 1963, BIOTRONIK has launched several innovations into the market--including remote monitoring with BIOTRONIK Home Monitoring® in 2000 and the world's first implantable cardioverter-defibrillators and implantable heart failure therapy devices with ProMRI® technology, approved for MR scanning, in 2012. In 2013, BIOTRONIK will be celebrating its fiftieth anniversary.
For more information: www.biotronik.com
Contact:
Manuela Schildwachter
Communications & PR Manager
BIOTRONIK SE & Co. KG
Woermannkehre 1
12359 Berlin
Tel. +49 (0) 30 68905 1466
Email: Email Contact
For more information: www.biotronik.com
Upon publication, please provide us with a copy.