Bimini Health Tech announced today it received FDA 510(k) clearance for its novel fat harvesting system, Dermapose Access (K200168).
|
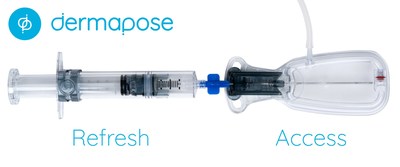
SOLANA BEACH, Calif., April 29, 2020 /PRNewswire/ -- Bimini Health Tech announced today it received FDA 510(k) clearance for its novel fat harvesting system, Dermapose Access (K200168). The Dermapose Access enables safe, quick, and easy fat harvesting. Designed for office use, the launch of the Dermapose Access system creates a new product category focused on simplifying fat removal.
"Dermapose Access overcomes one of the most challenging parts of fat transfer, safely removing fat," said Brad Conlan, CEO of Bimini Health Tech. "The product creates a totally controlled micro-environment for fat removal." "The Dermapose products were developed with safety and ease of use in mind. With FDA clearance of Dermapose Access, alongside Dermapose Refresh, we have expanded our product portfolio to bring safe and easy fat transfer to new settings such as a dermatologists procedure room." Added Mr. Conlan, "It's now simple for patients seeking natural procedures to volumize and rejuvenate their appearance." For information about Dermapose Access, please visit: www.hcp.dermapose.com/access/ To learn more about the entire Dermapose System, please visit: www.dermapose.com ABOUT BIMINI HEALTH TECH Bimini Health Tech is a global leader in the medical aesthetics market. The company develops and commercializes products that are elegant in their simplicity, yet impactful and proven in their aesthetic and therapeutic benefit. Since 2013, they have been developing innovative products to provide premium aesthetic care options to consumers and physicians alike. The Bimini Health Tech portfolio includes the brands Puregraft®, Dermapose®, and Kerastem®.
SOURCE Bimini Health Tech |