atHeart Medical, a medical device company dedicated to establishing the new standard of care for closure of atrial septal defects (ASD), today announced it has received approval for the start of the second phase of its ASCENT ASD U.S. Investigational Device Exemption (IDE) pivotal trial.
|
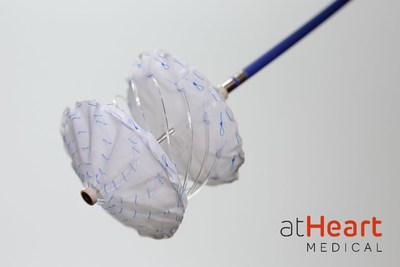
BAAR, Switzerland and SANTA CLARA, Calif., Feb. 8, 2022 /PRNewswire/ -- atHeart Medical, a medical device company dedicated to establishing the new standard of care for closure of atrial septal defects (ASD), today announced it has received approval for the start of the second phase of its ASCENT ASD U.S. Investigational Device Exemption (IDE) pivotal trial. The prospective, single-arm study is evaluating the safety and efficacy of the reSept™ ASD Occluder, the first occluder with a metal-free, bioresorbable frame, for the treatment of patients with clinically significant, isolated ASDs. Primary endpoints will be compared with established performance goals for previously FDA-approved transcatheter ASD occluders. "I am pleased the first phase of enrollment in our pivotal trial progressed smoothly and according to plan, this is an exciting milestone for the company," said Laurent Grandidier, CEO of atHeart Medical. "As we initiate the second phase, our team is focused on adding clinical sites across the U.S. and expanding internationally to include several enrolling sites in France. I commend the team's diligence to further validating the safety and efficacy of the reSept ASD Occluder, a critical step in our journey to evolve septal closure and provide a better solution for patients." The reSept ASD Occluder aims to address the limitations of current occluders which have metallic frames that can place patients at risk of complications associated with long-term presence of metal in the heart and may limit future transseptal interventions, such as mitral valve interventions. Initial clinical experience demonstrates positive safety and performance in the closure of the ASDs treated with the company's device.1 "The low-profile reSept Occluder is a dynamic system that allows for versatile physician control during the intervention, potentially adapting to the different patient anatomies to address ASDs," commented Dr. Scott Lim, Professor of Medicine & Pediatrics at the University of Virginia in Charlottesville, VA and a leading enrolling site in the trial. "Over time, reSept's metal-free frame resorbs, leaving a minimal implant behind. This is an exciting advancement that provides the potential to preserve future treatment options and potentially do better for our patients." About ASCENT-ASD Investigational Device Exemption (IDE) Trial About atHeart Medical About ASD
Media Contact:
SOURCE atHeart Medical |