SurGenTec, a privately held spine and orthopedic technology company dedicated to creating safe and intuitive solutions for surgeons and patients, announced today that it received U.S. Food and Drug Administration (FDA) 510(k) clearance for its 3D GraftRasp System, as well as key spine indications. This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20201001005710
BOCA RATON, Fla.--(BUSINESS WIRE)-- SurGenTec, a privately held spine and orthopedic technology company dedicated to creating safe and intuitive solutions for surgeons and patients, announced today that it received U.S. Food and Drug Administration (FDA) 510(k) clearance for its 3D GraftRasp System, as well as key spine indications.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20201001005710/en/
3D GraftRasp System (Photo: Business Wire)
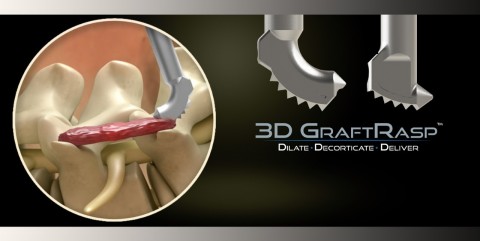
The 3D GraftRasp System is the only device to receive FDA clearance that allows for both decortication of bone and the delivery of autograft, allograft or synthetic bone graft. It is approved for general orthopedic use and spine procedures. “In spine procedures, the transverse processes and facet joints may now be targeted in a more efficient and effective surgical technique pioneered by SurGenTec,” says Founder and CEO Travis Greenhalgh. The 3D GraftRasp System is ideal for use in posterolateral or intertransverse lumbar fusion cases.
Posterolateral fusion has long been a standard of care to treat lumbar spine disorders and remains one of the most commonly performed procedures. In open posterolateral lumbar fusion surgery, the transverse processes and facet joints are routinely decorticated and bone graft is packed in lateral trench-like gutters to help achieve fusion. In minimally invasive cases, this region is typically neglected due to challenges accessing the anatomy. SurGenTec’s new 3D GraftRasp System allows physicians to easily access the anatomy while making less invasive incisions and offering the same standard of care as with an open lumbar fusion.
The 3D GraftRasp system features instruments including straight or curved rasps, specific to the surgical site anatomy. The rasps feature disposable foot plates with conical teeth that help to ensure optimal decortication. 3D GraftRasp is intended to be used in conjunction with the GraftGun® delivery system to facilitate controlled bone graft delivery to the surgical site. Autograft, allograft or synthetic bone graft is extruded with accuracy through an aperture in the rasp directly to the decorticated area, which instills confidence that bone graft is placed precisely where fusion is desired. The 3D GraftRasp System was designed for synergy between the GraftRasp instrument and GraftGun. For ease of use and saving time the GraftGun tubes are pre-loaded with OsteoFlo® NanoPutty®(Synthetic), ViMax® fiber cellular matrix, or Ossify™ DBM putty and Ossify™ cortical fibers. The universal GraftGun kit may also be utilized to allow surgeons the freedom to load the bone graft of their choice.
“FDA clearance of the 3D GraftRasp System expands our treatment modalities in the spine market. This is an exciting time for our company and for physicians who need intuitive instruments to treat their patients,” said Andrew Shoup, SurGenTec’s COO. SurGenTec is currently launching several unique product lines focused on physician needs and the 3D GraftRasp System fills a void in spine surgery that’s been overlooked for years. “Physicians want efficiency and better outcomes, while patients want a better chance to resume a lifestyle that makes them happy and pain free,” said Shoup.
The 3D GraftRasp System is protected by more than 10 patents with more applications pending. SurGenTec plans to release iterations for other general orthopedic uses such as large-joint and the foot/ankle markets. The company is releasing the 3D GraftRasp System immediately and will be hosting physician training sessions in its new BioSkills Lab in Boca Raton, FL. The company has the ability to host live wet-lab training sessions or physicians may attend remotely via video conference.
About SurGenTec, LLC
SurGenTec is focused on development and marketing of unique, innovative products for orthopedic and spine markets. They currently have numerous products cleared by the FDA including their recently approved OsteoFlo NanoPutty synthetic bone graft. SurGenTec’s products are approved in the US and internationally. For more information on the Company’s products or training please visit www.surgentec.com or contact customerservice@surgentec.com or call 561-990-7882.
View source version on businesswire.com: https://www.businesswire.com/news/home/20201001005710/en/
Source: SurGenTec, LLC








