Highlights: Statistically significant reduction in MRSA superbug bacterial load and higher percentage of wound contraction with RECCE ® 327 as compared to Soframycin in rat model for topical burns Study reinforces the potential of RECCE ® 327 against drug-resistant superbugs
Highlights:
- Statistically significant reduction in MRSA superbug bacterial load and higher percentage of wound contraction with RECCE® 327 as compared to Soframycin in rat model for topical burns
- Study reinforces the potential of RECCE® 327 against drug-resistant superbugs
SYDNEY, Australia, April 23, 2020 (GLOBE NEWSWIRE) -- Recce Pharma Ltd (ASX: RCE) (Company), the company developing a new class of broad-spectrum synthetic antibiotics, today announced positive data showing significant in-vivo antibacterial activity against Methicillin-Resistant Staphylococcus aureus (MRSA superbug) in rats with topical burns treated with its lead compound RECCE® 327.
“We are greatly encouraged by the data because it further reinforces RECCE® 327 is potent and keeps on working with repeated efficacy against topical pathogens and superbugs at different dosing levels,” said Dr John Prendergast, Non-Executive Chairman. “Recce’s synthetic antibiotic out-performed the best in class antibiotic Soframycin showing it could be a potential alternative treatment for resistant Staphylococcus aureus, one of the most common bacterial infections in humans.”
The study was conducted by an independent Contract Research Organization to assess the dose-dependency of RECCE® 327 and in-vivo antibacterial activity against MRSA in rats with topical burns. It met its primary endpoints which were a reduction in bacterial load in wound and percentage of wound contraction, evaluated on the fourth day following dosing.
RECCE® 327 was effective in reducing bacterial load within a wound and showed enhanced wound contraction in comparison to the best in class - Soframycin. RECCE® 327 showed repeated efficacy at different dosing levels on topical skin conditions even at low doses. This additional antibacterial efficacy data will be presented to a leading Australian teaching hospital for their anticipated clinical trial considerations.
Bacterial Count Assessment
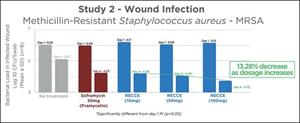
Five groups of eight rats each showed RECCE® 327 performed better in all instances compared to those who received the optimum dose Soframycin treatment or no treatment. RECCE® 327 continued to show efficacy at different dose levels with significant reduction in bacterial count in the infected wound when compared to the vehicle control (p<0.05). As dosage increased from 10mg to 100mg, there was a further 13.28% decrease in bacterial load.
A photo accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/9a7a8146-86a6-4e8d-ba71-325ceaa7227e
| Log10 CFU/Swab (Mean ± SD)(n=8) | |||||
| Group | Treatment | Day 1 PI** | Day 4 PI** | % Change | |
| 1 | Burn wound With infection | 6.05 | 5.07 | 16.2% | |
| 2 | Burn wound With infection + Marketed Drug [30mg] (Soframycin***) | 6.00 | 4.23* | 29.5% | |
| 3 | Burn wound With infection+ RECCE [10 mg] | 6.17 | 4.29* | 30.5% | |
| 4 | Burn wound With infection+ RECCE [50 mg] | 6.09 | 4.08* | 33.0% | |
| 5 | Burn wound With infection+ RECCE [100 mg] | 6.15 | 3.72* | 39.5% | |
*significantly different from day 1 PI [p<0.05]
** PI – Post Infection
*** Topically marketed antibiotic for the treatment of bacterial infections in burns and wounds
RECCE® 327 showed a significant dose-dependent antibacterial effect when compared to the vehicle control (p<0.05). In this study Soframycin applied twice daily at optimum therapeutic dose whereas a once daily application of RECCE® 327 demonstrated antibacterial efficacy reinforcing RECCE® 327 may be a more potent antibiotic without additional toxicity considerations associated with similar doses of Soframycin.
Wound Contraction/Healing Assessment
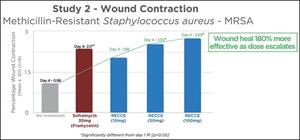
RECCE® 327 was further assessed in a wound contraction study. RECCE® 327 showed significant dose-dependent wound healing activity when compared to the vehicle control (p<0.05). Additionally, RECCE® 327 was 180% more effective in wound healing as the dose escalated in comparison to the group that received no treatment.
A photo accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/45f250bf-68d7-4e8e-ae35-41ebe4647141
| Percentage wound contraction (Mean ± SD)(n=8) | ||
| Group | Treatment | Day 4 PI |
| 1 | Burn wound With infection | 0.96 ± 0.54 |
| 2 | Burn wound With infection + Market Drug [30 mg] (Soframycin) | 2.17 ± 0.81* |
| 3 | Burn wound With infection+ RECCE [10 mg] | 1.89 ± 0.94ns |
| 4 | Burn wound With infection+ RECCE [50 mg] | 2.55 ± 0.49* |
| 5 | Burn wound With infection+ RECCE [100 mg] | 2.69 ± 1.05* |
ns Not significantly different from day 1 PI [p>0.05].
* Significantly different from day 1 PI [p<0.05].
Staphylococcus aureus (S. aureus) is a Gram-positive bacteria found on the skin and mucous membranes. S. aureus is the most dangerous of all of the many common staphylococcal bacteria. This bacteria often causes skin infections; however, it can also cause pneumonia, bone infections, meningitis and other invasive infections.1 Patients with MRSA have significantly longer hospital stays and are estimated to be 64% more likely to die than people with a non-resistant form of the infection.2
1https://www.ncbi.nlm.nih.gov/books/NBK441868/
2 https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance
About Recce Pharmaceuticals Ltd
Recce Pharmaceuticals Ltd (ASX: RCE) is pioneering the development and commercialization of a New Class of Synthetic Antibiotics with Broad Spectrum activity designed to address the urgent global health problem of antibiotic resistant superbugs.
Recce antibiotics are unique – their potency does not diminish even with repeated use, which is a common failure associated with existing antibiotic use and the resulting emergence of resistant superbugs.
Patented lead candidate RECCE® 327, wholly owned and manufactured in Australia, has been developed for the treatment of blood infections and sepsis derived from E. coli and S. aureus bacteria – including their superbug forms.
The FDA has awarded RECCE® 327 Qualified Infectious Disease Product designation under the Generating Antibiotic Initiatives Now (GAIN) Act – labelling it for Fast Track Designation, plus 10 years of market exclusivity post approval.
Recce wholly owns its automated manufacturing, ready to support first-in-human clinical trials. Recce’s anti-infective pipeline seeks to exploit the unique capabilities of RECCE® technologies targeting synergistic, unmet medical needs.
| Executive Director | Media & Investor Relations (AU) | Media & Investor Relations (USA) |
| James Graham | Andrew Geddes | Meredith Sosulski, PhD |
| Recce Pharmaceuticals, Ltd. | CityPR | LifeSci Communications |
| +61 (02) 8075 4585 | +61 (02) 9267 4511 | +1 929 469 3851 |
| james.graham@recce.com.au | ageddes@citypublicrelations.com.au | msosulski@lifescicomms.com |