Penumbra, Inc. (NYSE: PEN), a global healthcare company focused on innovative therapies, today announced U.S. commercial availability of the Indigo® System Lightning™ 7. Lightning 7 expands Penumbra’s offering of the Indigo Aspiration System with Intelligent Aspiration for mechanical thrombectomy and is designed for single session arterial thrombus removal.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20210318005280/en/
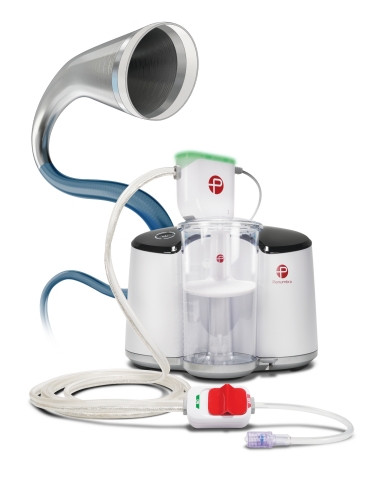
Lightning™ 7 – Intelligent Aspiration Powered by Penumbra ENGINE™ (Photo: Business Wire)
Offering a new option for physicians to address arterial thrombus removal, the Indigo System Lightning 7 combines the new Indigo System CAT™7 Aspiration Catheter with Lightning Intelligent Aspiration powered by Penumbra ENGINE®. CAT7 is a high power, low profile catheter that features laser-cut hypotube technology and circumferential sweep designed for dependable delivery and maximized clot extraction. The Indigo System’s proprietary Separator™ technology is also available with Lightning 7 (Separator 7) and is designed to enable unobstructed aspiration for the duration of the procedure. Lightning Intelligent Aspiration features Penumbra’s proprietary clot detection technology that enables the operator to identify thrombus location and is also designed for blood loss reduction.
“My experience with Lightning 7 suggests that it streamlines clot removal in the peripheral arterial vasculature with low profile access, excellent trackability, and similar power to the CAT8 Penumbra catheter that was previously used for this application. The torquability makes vessel navigation much easier,” said Dr. Christopher Metzger, System Chair of Clinical Research for Ballad Health and Medical Director of Cardiac and Peripheral Cathlabs, Ballad Health, Holston Valley Medical Center, Kingsport, Tennessee. “Penumbra continues to forge ahead with yet another game-changing technology that has the potential to increase single-session thrombus removal and thereby may help improve outcomes.”
“Today in the United States, we believe the number of patients presenting to the hospital with arterial thrombus is significantly higher than patients presenting with thrombus elsewhere in the body. Conditions involving arterial thrombus are often associated with high amputation rates, and mortality as a result. We are proud to introduce another important technology to enable physicians to reduce the need for thrombolysis, which in turn can reduce demand for ICU beds,” said Adam Elsesser, president and chief executive officer, Penumbra. “We are excited by the early experience with Lightning 7 that suggests we can help physicians to simplify arterial thrombus removal with more single-session results.”
About Indigo System with Lightning Intelligent Aspiration
The Indigo System with Lightning is an intelligent aspiration system powered by Penumbra ENGINE and was introduced in July 2020. Lightning Aspiration Tubing has dual pressure sensors for real-time blood flow monitoring. The built-in microprocessor features a proprietary thrombus removal algorithm that automatically controls a valve in the tubing to provide continuous or intermittent aspiration. This unique mechanism of action helps optimize thrombus removal procedures by differentiating between thrombus and blood. The system is designed to aspirate continuously when in thrombus and intermittently in patent flow. When the Indigo aspiration catheter is placed at the clot and in patent flow, Lightning is designed to automatically register a change in pressure and to minimize flow with intermittent aspiration. Throughout the case, Lightning provides procedural feedback via audiovisual cues. Lightning’s thrombus removal algorithm is designed to initiate automatic valve clicking when it senses patent flow. With automatic valve control, Lightning is designed to help reduce blood loss and allow the physician to focus on optimizing thrombus removal procedures. The Indigo System with Lightning Intelligent Aspiration is available in the United States in the following configurations: Lightning 12, Lightning 8 and Lightning 7.
The Indigo System with Lightning Intelligent Aspiration and Separators is indicated for the removal of fresh, soft emboli and thrombi from the peripheral arterial and venous systems, and for treatment of pulmonary embolism.
Important Safety Information
Additional information about Penumbra’s products can be located on Penumbra’s website at http://www.penumbrainc.com/healthcare-professionals. Prior to use, please refer to Instructions for Use for complete product indications, contraindications, warnings, precautions, potential adverse events and detailed instructions for use.
About Penumbra
Penumbra, Inc., headquartered in Alameda, California, is a global healthcare company focused on innovative therapies. Penumbra designs, develops, manufactures and markets novel products and has a broad portfolio that addresses challenging medical conditions in markets with significant unmet need. Penumbra sells its products to hospitals and healthcare providers primarily through its direct sales organization in the U.S., most of Europe, Canada and Australia, and through distributors in select international markets. Penumbra, the Penumbra P logo, Indigo, CAT, Separator, Lightning, and Penumbra ENGINE are trademarks of Penumbra, Inc. For more information, visit www.penumbrainc.com and connect on Twitter and LinkedIn.
Forward-Looking Statements
Except for historical information, certain statements in this press release are forward-looking in nature and are subject to risks, uncertainties and assumptions about us. Our business and operations are subject to a variety of risks and uncertainties and, consequently, actual results may differ materially from those projected by any forward-looking statements. Factors that could cause actual results to differ from those projected include, but are not limited to: the impact of the COVID-19 pandemic on our business, results of operations and financial condition; failure to sustain or grow profitability or generate positive cash flows; failure to effectively introduce and market new products; delays in product introductions; significant competition; inability to further penetrate our current customer base, expand our user base and increase the frequency of use of our products by our customers; inability to achieve or maintain satisfactory pricing and margins; manufacturing difficulties; permanent write-downs or write-offs of our inventory; product defects or failures; unfavorable outcomes in clinical trials; inability to maintain our culture as we grow; fluctuations in foreign currency exchange rates; and potential adverse regulatory actions. These risks and uncertainties, as well as others, are discussed in greater detail in our filings with the Securities and Exchange Commission, including our Annual Report on Form 10-K for the year ended December 31, 2020 filed with the SEC on February 23, 2021, and our Quarterly Reports on Form 10-Q. There may be additional risks of which we are not presently aware or that we currently believe are immaterial which could have an adverse impact on our business. Any forward-looking statements are based on our current expectations, estimates and assumptions regarding future events and are applicable only as of the dates of such statements. We make no commitment to revise or update any forward-looking statements in order to reflect events or circumstances that may change.
Source: Penumbra, Inc.
View source version on businesswire.com: https://www.businesswire.com/news/home/20210318005280/en/
Contacts
Investor Relations:
Penumbra, Inc.
investors@penumbrainc.com
510-995-2461
Media Relations:
Betsy Merryman
Merryman Communications
betsy@merrymancommunications.com
310-560-8176
Source: Penumbra, Inc.