AptarGroup, Inc. announced that its Activ-Blister™ packaging solution for oral solid dose drug delivery was recently approved by the U.S. Food and Drug Administration for a HIV prevention medicine..
CRYSTAL LAKE, Ill.--(BUSINESS WIRE)-- AptarGroup, Inc. (NYSE: ATR), a global leader in consumer dispensing, active packaging and drug delivery solutions, today announced that its Activ-Blister™ packaging solution for oral solid dose drug delivery was recently approved by the U.S. Food and Drug Administration (FDA) for a HIV prevention medicine.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20191022005932/en/
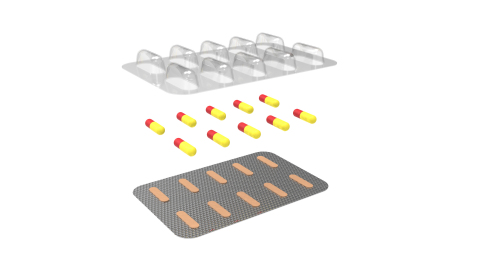
Photo: Aptar’s Activ-Blister™ packaging solution
The oral solid dose drug was developed by a leading pharmaceutical company in the HIV treatment and prevention space, and represents the first FDA approval of Aptar’s proprietary Activ-Blister ™ packaging solution and proprietary application process developed by Aptar CSP Technologies.
The system protects oral solid drug products with a 3-Phase Activ-Polymer™ solution that is fully integrated into the blister package. This 3-Phase Activ-Polymer™ technology, which can be customized specifically for the drug developer’s formulation, offers a broad spectrum of specific drug protection including moisture adsorption, and oxygen and odor scavenging. The technology can also scavenge volatile organic compounds (VOCs) and emit aromas.
Aptar’s portfolio of 3-Phase Activ-Polymer™ protective solutions helps biotech and pharmaceutical partners meet specific drug long-term and in-use stability requirements while maintaining therapeutic efficacy, all in a patient-friendly packaging configuration.
“I am very pleased to announce this recent FDA approval of our proprietary Activ-Blister™ technology,” commented Stephan Tanda, Aptar’s President and CEO. “This is a significant step that further validates the expanding portfolio of solutions offered by Aptar CSP Technologies. We will continue to leverage our proprietary 3-Phase Activ-Polymer™ technology to help our customers with unique protective formulations that derisk their drug development process and help strengthen their own offerings. The ultimate result is that we are creating meaningful solutions that help improve and save lives.”
About Aptar
Aptar CSP Technologies is part of AptarGroup, Inc., a leading global supplier of a broad range of innovative dispensing, sealing and active packaging solutions for the beauty, personal care, home care, prescription drug, consumer health care, injectables, food and beverage markets. Aptar uses insights, design, engineering and science to create innovative packaging technologies that build brand value for its customers, and, in turn, make a meaningful difference in the lives, looks, health and homes of people around the world. Aptar is headquartered in Crystal Lake, Illinois and has over 14,000 dedicated employees in 18 different countries. For more information, visit www.csptechnologies.com or www.aptar.com.
View source version on businesswire.com: https://www.businesswire.com/news/home/20191022005932/en/
Contacts
Investor Relations Contact:
Matt DellaMaria
AptarGroup, Inc.
+1 815 477 0424
matt.dellamaria@aptar.com
Media Contact:
Badre Hammond
Aptar CSP Technologies
+1 845 639 3399
badre.hammond@aptar.com
Source: AptarGroup, Inc.
View this news release and multimedia online at:
http://www.businesswire.com/news/home/20191022005932/en







